Eco-friendly Coating of Natural Zeolite with Metallic Gold, and Characterization of the Resulting Products
Salprima Yudha S1 , Morina Adfa1 and Aswin Falahudin2
, Morina Adfa1 and Aswin Falahudin2
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Bengkulu, Indonesia.
2Jalan W.R, Supratman, Kandang Limun, Kota Bengkulu 38371A, Indonesia.
Corresponding Author E-mail: salprima@unib.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340160
Callophyllum Inophyllum L; Coating; Metallic Gold; Natural Zeolite
Download this article as:| Copy the following to cite this article: Yudha S, Adfa M, Falahudin A. Eco-friendly Coating of Natural Zeolite with Metallic Gold, and Characterization of the Resulting Products. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Yudha S, Adfa M, Falahudin A. Eco-friendly Coating of Natural Zeolite with Metallic Gold, and Characterization of the Resulting Products. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=43084 |
Introduction
There are many reports on the modification of supporting materials to enhance the stability of gold nanoparticles, which have been developed for various purposes. For instance, calixarene-functionalized gold nanoparticles, which are potentially useful in colorimetric sensors involving Cu2+ and Pb2+ ions, have been prepared recently.1 However, zeolites also play an important role as supporting materials. For example, gold nanoparticles loaded onto zeolite can be used for laser mass spectrometry,2 and modified zeolite- supported gold nanoparticles are effective as an antibacterial agent or a peroxide sensor.3-4 Solid- supported gold nanoparticles are also useful as heterogenous or quasi-homogenous catalysts; zeolite-encapsulated gold nanoparticles are effective for the oxidation of bioethanol.5 and the in situ growth of Au nanoparticles (AuNPs) on Fe O via a cysteine-linked approach, and their use as a catalyst for 4-nitrophenol reduction, has been reported recently.6
Moreover, there has been a rapid increase in the use of semiconductor materials such as ZnO and TiO2 as supporting materials. The authors of one report claim that the photocatalytic efficiencies of Au/TiO2 and Pt/TiO2 are higher than those of Pd/ TiO2 with respect to the degradation of an aqueous solution of acid green 16.7 Recently, various organic dyes—including Methylene Blue, Methyl Orange, Congo Red, Rhodamine B (RhB), and Malachite Green—have been reductively degraded using an Au/CeO2–TiO2 nanohybrid.8 Furthermore, the photocatalytic activity of an Au/TiO2 nanocomposite, which can be used to remove RhB, has been enhanced by preparing it by chemical reduction using hydrazine hydrate.9 These findings have been corroborated by other research: a mesoporous Au–TiO2 nanocomposite, prepared by a simple hydrolytic spray method, effectively decolorized aqueous RhB under visible light irradiation (λ > 420 nm).10 The catalytic degradation of RhB using Au loaded onto TiO2 nanotube arrays is enhanced by sunlight,11 and titania (TiO2)- and zincite (ZnO)-supported gold nanoparticles have photodegradation activities with respect to RhB under UV-A light irradiation.12 Another example is the use of TiO2 doped with various metals.13 Other results show that multiwalled carbon nanotubes with hetero-oligophenylene-stabilized gold nanoparticles effectively degrade RhB in visible light.14 Recently, we have reported the environmentally friendly (green) synthesis of silver and gold nanoparticles using plant extracts; for example, we have synthesized gold nanoparticles using an extract from Calophyllum inophyllum L. leaves. Analysis revealed that spherical gold nanoparticles with an average diameter of 27 nm have a surface plasmon resonance peak at 539 nm.15
The existing research has stimulated our interest in developing a green method of coating natural zeolite with metallic gold, and characterizing the resulting products. We believe our research will significantly contribute to the development of competitive green substances that are compatible with existing materials.
Experimenital
Material and Methods
A natural zeolite was collected from Taba Penanjung, Bengkulu, Indonesia, and the C. Inophyllum L leaves were taken from Bengkulu City, Indonesia. The gold ion precursor (HAuCl4•3H2O) was purchased from Sigma-Aldrich (Germany). Demineralized water was used throughout.
General Procedure
The natural zeolite was ground using a mortar until it was able to pass through a 200 mesh sieve (75 µM). The fine particles were washed three times with demineralized water and dried at 100 °C for 2 h (we referred to the product as pre-treated natural zeolite). The pre-treated natural zeolite (10 g) was immersed in 50 mL of 30% H2O2 and kept at room temperature for 40 h in the dark. The mixture was heated at 50 °C for 1 h and 100 °C for 1 h, then centrifuged at 4500 rpm for 5 min. to obtain a solid. The solid material was washed with demineralized water and centrifuged up to three times at 4500 rpm for 5 min. (we referred to the product as H2O2-treated natural zeolite). The H2O2-treated natural zeolite (1 g) was then immersed in 0.01 M HAuCl4 solution for 24 h. The C. Inophyllum L. Leaf extract was prepared by heating the air-dried leaves (2 g) in hot demineralized water (100 Ml) for 30 minute. The fresh leaf extract (25 Ml) was added dropwise to the suspension of HAuCl4/zeolite while stirring magnetically. The reaction mixture was aged at room temperature for 48 h, then heated until the solvent had completely evaporated. The solid black material was then placed in a crucible and heated in a furnace at 300 °C for 1 h to produce a solid dry material (Figure. 1).
Results and Discussion
Figure. 1 is a flow diagram illustrating the process used to coat the natural zeolite with metallic gold. The overall color changed from solid gray (natural zeolite) to deep brown (gold-coated natural zeolite). H2O2 was used to remove organic compounds trapped in the natural zeolite.
 |
Figure 1: Treatment flow to from metallic gold-decorated natural zeolite; (a) raw natural zeolite,(b)natural zeolite powder 200 mesh, (c) H2O2-treated natural zeolite powder,(d)metallic gold-decorated natural zeolite Click here to View figure |
The immersion of the zeolite powder in HAuCl4 solution for 24 h ensured the impregnation of gold ions. The addition of C. inophyllum L. leaf extract to the reaction mixture reduced the Au3+ to Au0, which was signaled by a change in the color of the reaction mixture from yellow to deep brown. The water was evaporated to produce a gel, which was calcined at 300°C for 1 h to remove the organic material residue from the solid material. We characterized the obtained materials ((1b), (1c), and (1d)) by X-ray diffraction (XRD), as shown in Figure. 2.
 |
Figure 2: XRD pattern of the (a) pre-tretated natural zeolite (b) H2O2-tretated natural zeolite (c) Gold metallic-coated natural zeolit Click here to View figure |
The pre-treated natural zeolite (Fig 2a) exhibited peaks at 2θ = 20.06°, 23.96°, 26.86°, 35. 32°, and 62.28°, and many other peaks indicating that the zeolite was in the amorphous phase. The peaks indicated that the clay comprised montmorillonite with its characteristic peaks at 2θ = 20.06°, 35.32°, and 62.28°. The other peaks were attributable to impurities corresponding to feldspar (23.96°) and quartz (26.86°). These results are corroborated by those for other Na-bentonite type zeolites.16 However, the H2O2-treated natural zeolite (Fig 2b) exhibited peaks at 2θ = 19.86°, 26.64°, 36.96°, and 61.94°. The peak for feldspar (23.96°) disappeared during the treatment. The final material was also characterized by XRD, and the results are shown in Fig 2c. The new peaks at 2θ = 37.66°, 44.00°, 64.22°, and 77.18° were attributable to metallic gold, and are corroborated by similar results from other reports.17 Although there was no precise evidence showing the coating sites (on the surface or in the zeolite matrix), it was clear that the zeolite had been coated with metallic gold during the treatment.
We conducted further investigations using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) to obtain more information about the morphology and elemental composition of the target materials, as shown in Figure. 3.
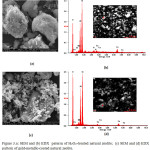 |
Figure 3.a: SEM and (b) EDX paterrn of H2O2-treated natural zeolite; (c) SEM and (d) EDX pattern of gold-metallic-coated natural zeolite Click here to View figure |
According to Fig. 3 (a), the H2O2-treated natural zeolite comprised bulk particles with irregular shapes. Fig. 3 (b) shows the elements present in the H2O2-treated natural zeolite, according to the EDX analysis. In the EDX spectra of the natural zeolite, peaks appeared at 0.50, 1.50, and 1.80 keV corresponding to the binding energies of O, Al, and Si, respectively. These peaks approximately correspond to those reported for the TMA-A zeolites synthesized using hydrothermal methods.18 Further treatment of the H2O2-treated natural zeolite with a solution of gold ions, followed by reduction using C. inophyllum L. leaf extract, produced more regularly shape particles, as shown in Fig. 3(c). Although element mapping using EDX (Fig. 3(d)) did not definitively prove the presence of gold in the zeolite, the XRD results did suggest that metallic gold was present.
As reported in similar previous reports on zeolite-supported gold nanoparticles, the current metallic gold-coated natural zeolite has potential for use in various applications such as photocatalytic studies on RhB, reactive Red-198, and chlorophenols,19-120 the selective photooxidation of aromatic alcohols by irradiation with visible light,17 and the amperometric biosensing of spermidine.18
Conclusion
We successfully coated a natural zeolite with metallic gold using a green method that utilizes a leaf extract as the reducing agent. We obtained products that have potential as materials for various applications such as catalysts, antibacterial agents, agents for the photocatalytic degradation of organic pollutants, etc. Research in this field continues in our laboratory.
Acknowledgement
S.Y.S and M.A thank Direktorat Riset dan Pengabdian Masyarakat, Kementerian Riset, Teknologi, dan Pendidikan Tinggi, the Republic of Indonesia for research funding. A.F. thanks the head of the Department of Chemistry at the University of Bengkulu for the opportunity as a Research Assistant at the Division of Inorganic Chemistry.
References
- Gunupuru, R.; Maity, D.; Bhadu, G.R.; Chakraborty, A.; Srivastava, D.N.; Paul, P. J. Chem. Sci. 2014, 126 (3) 627 – 635.
CrossRef - Yang, M.; Fujino, T. Chem. Phys. Lett. 2014, 592, 160 –163.
CrossRef - Lima, E.; Guerra, R.; Lara, V.; Guzmán, A. Chemistry Central J. 2013, 7 (11) 7 pages, DOI: 10.1186/1752-153X-7-11.
CrossRef - Ren, L.; Dong, J.; Cheng, X.; Xu, J.; Hu, P. Microchimica Acta, 2013, 180 (13) 1333 –1340.
CrossRef - Mielby, J.; Abildstrøm, J.O.; Wang, F.; Kasama, T.; Weidenthaler, C.; Kegnæs, S. Angewadnte Chemie Intl. Ed., 2014, 53 (46) 12513–12516.
- Cao, S.-W.; Fang, J.; Shahjamali, M.M.; Wang, Z.; Yin, Z.; Yang, Y.; Boey, F.Y.C.; Barber, J.; Loo, S.C.J.; Xue, C. Cryst. Eng Comm. 2012, 14, 7229–7235.
CrossRef - Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Water Research, 2004, 38 (13) 3001– 3008.
CrossRef - Saikia, P.; Miah, A.T.; Das, P.P. J. Chem. Sci. 2017, 129 (1), 81 – 93.
CrossRef - Zhang, D. Polish J. Chem. Tech.2012, 14 (2) 42 – 48.
CrossRef - Zhou, M., Zhang, J.; Cheng, B.; Yu, H. Intl. J. Photoenergy, 2012, Article ID 532843, 10 pages, http://dx.doi.org/10.1155/2012/532843.
CrossRef - Sun, S.; Chen, C.; Sun, J.; Peng, Q.; Lü, K.; Deng, K. Procedia Env. Sci. 2013, 18, 620 – 624.
CrossRef - Alshammari, A.; Bagabas, A.; Assulami, M.; Arabian J. Chemistry, Available online November 10, 2014. http://dx.doi.org/10.1016/j.arabjc.2014.11.013.
CrossRef - Tan, Y.N.; Wong, C.L.; Mohamed, A.R. ISRN Materials Science, 2011, Article ID 261219, 18 pages, doi:10.5402/2011/261219.
CrossRef - Kaur, S.; Bhalla, V.; Kumar, M.; ACS Applied Materials & Interfaces, 2015, 7 (30), 16617 – 16624.
CrossRef - Yudha S., S.; Mardlia, Z.A.; Angasa, E.; Suharto, T. E.; Nishina, Y. In Proceeding The 9th Joint Conference on Chemistry, 2014, Semarang, Indonesia, November 12-13, 2014.
- Zhironga, L.; Uddin, M.A.; Zhanxuea, S.; Spectrochimica Acta Part A, 2011, 79, 1013 – 1016.
CrossRef - Zhang, X.; Ke, X.; Zhu, H. Chem. Eur. J. 2012, 18, 8048 – 8056.
CrossRef - Chauhan, N.; Jain, U.; Gandotra, R.; Hooda, V. Electrochimica Acta, 2017, 230, 106 –115.
CrossRef - Reddy, G.R.; Balasubramanian, S.; Chennakesavulu, K. RSC Adv., 2015, 5, 81013 – 81023.
CrossRef - León, E.R.; Rodríguez, E.L.; Beas, C.R.; Plascencia-Villa,G.; Palomares, R.A.I. J. Nanomaterials, 2016, Article ID 9541683, 10 pages, http://dx.doi.org/ 10.1155/2016/9541683.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









