Cooxidation not to be Confused with Catalysis: A Chemical Education Article to Physical-Organic Chemists
R. Sanjeev1 , D. A. Padmavathi2 and V. Jagannadham2
, D. A. Padmavathi2 and V. Jagannadham2
1Department of Chemistry, Geethanjali College of Engineering and Technology, Cheeryal-501301, India.
2Department of Chemistry, Osmania University, Hyderabad 500007, India.
Corresponding Author E-mail: jagannadham1950@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340159
Two substrates (A) and (B) are oxidized separately by an oxidant (Oxi) with the rate constants k1 and k2 and they are oxidized taken together (A + B) under similar conditions with a rate constant k3, if the value of k3 = (k1 + k2), then it is said to be an example of two reactions “going parallel”. If the value of k3 >>>> (k1 + k2), then the redox process is termed as “co-oxidation” (Hasan and Roček 1972, JACS). In this process in the mixture the two substrates are oxidized synchronously by a direct three electron transfer route if the oxidant happens to be Cr(VI) and by a direct four electron transfer route if the oxidant happens to be Mn(VII) (Jagannadham et.al. 1986, Oxidation Communications). It was realized that the essential condition of the synchronous oxidation of two substrates A and B is that one substrate must have two functional groups and the other must have one functional group or vice-versa. The compound with two functional groups must be a good chelating agent with the metal ion oxidant. A substrate (A) is oxidized by an oxidant (Oxi) with a rate a constant k4 and is oxidized in presence of a catalyst (Cat) with a rate constant k5, if k5 > k4 the redox process is termed as “catalyzed process”. It is to be noted that in the catalytic process the catalyst (Cat) is not oxidized and its concentration does not change during the reaction. It only increases the rate of oxidation with lower activation energies. If k5 = k4 it is to be understood that there is “no catalysis”. If k5 < k4 it is to be understood that the catalyst is called a negative catalyst or “inhibitor” and the reaction goes with higher activation energy. In this paper a lucid description is given for the two processes “co-oxidation” and “catalysis” with putative examples.
KEYWORDS:Synchronous Oxidation; putative; catalysis
Download this article as:| Copy the following to cite this article: Sanjeev R, Padmavathi D. A, Jagannadham V. Cooxidation not to be Confused with Catalysis: A Chemical Education Article to Physical-Organic Chemists. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Sanjeev R, Padmavathi D. A, Jagannadham V. Cooxidation not to be Confused with Catalysis: A Chemical Education Article to Physical-Organic Chemists. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42721 |
Introduction
Hasan and Roček were the first to report a direct synchronous three electron oxidation process where in isopropyl alcohol and oxalic acids were oxidized1. Later several publications appeared from his laboratory2-18. Sequel to Roček’s discovery1 of one step three electron oxidations several researchers reported numerous studies in this area. Quoting them in this article is beyond the scope and limit of this article. However to one of the authors of this article the situation warrants to quote our own examples of oxidation of aliphatic esters in presence of oxalic acid again by a synchronous three electron transfer route19, and oxidation of iso-propanol and lactic acid by Cr(VI)20 and a mixed substrate system of an aldehyde and an alcohol21. Another interesting study by Roček on three electron oxidation is the intra-molecular cooxidation of a single substrate having three functional groups such as 2,7-dihydroxyheptanoic acid by chromic acid13. 2,7-dihydroxyheptanoic acid is a substitute of two substrates one with two functional groups and the other with one functional group. Though not effective as 2,7-dihydroxyheptanoic acid oxidation, a similar study was also reported from our laboratory where in oxidation of glycerol which has three hydroxyl groups by chromic acid did take place by a three electron transfer route22. Our study was further extended to permanganate ion oxidations where in iso-propanol was oxidized by permanganate ion in presence of several bi-functional compounds by synchronous four electron transfer route23.
The end product of the oxidant in three electron oxidations by Cr(VI) is Cr(III). As Cr(III) is stable it does not undergo any further reduction. But the situation in the four electron transfer reaction by Mn(VII) is different. The end product of Mn(VII) is Mn(III) which is known to be reasonably a good oxidant ending with the formation of Mn(II) as the stable end product24. In the previous work23 from our laboratory care is taken so that the similar conditions were adopted where in the oxidation of either iso-propanol or the bifunctional compounds by Mn(III) was negligible.
Catalysis is the process that increases the rate of a chemical reaction due to the participation of a third substance called a catalyst. The catalyst is not consumed in the course of the catalyzed reaction and can continue in several cycles until the completion of the reaction. Very often only micro-molar quantities of the catalyst are required in principle25. In catalytic process the catalyst is not oxidized or reduced.
Hence the Process of Co-Oxidation Should not be Confused with Catalysis
Discussion
Before understanding the difference between cooxidation and catalysis it is noteworthy to discuss the oxidations of related compounds by Cr(VI) and Mn(VII) in detail.
Oxidation of iso-propanol by Cr(VI)
Roček and Krupička26a and Wiberg and Schaefer 26b reported the oxidation of iso-propanol by chromic acid. The reaction was shown to undergo via a chromate ester which is formed in a reversible step and then decomposes in a rate determining step to give acetone as shown in the following mechanism (Scheme 1).
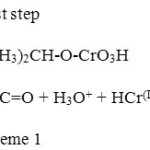 |
Scheme 1 Click here to View scheme |
Oxidation of Oxalic Acid by Cr(VI)
Oxidation of oxalic acid was studied by Bakore and Jain27 and later by Hasan and Roček28. The reaction involves a cyclic transition state between chromic acid and oxalic acid which later decomposes in a rate determining step to give CO2 as the end product. The mechanism is shown in Scheme 2.
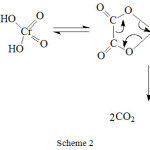 |
Scheme 2 Click here to View scheme |
Oxidation of A Mixture of Iso-Propanol and Oxalic Acid by Cr(VI)
Hasan and Roček for the first time reported a direct synchronous three electron oxidation process where in isopropyl alcohol and oxalic acids were both oxidized1. They strongly advocated the reaction to be a direct three electron transfer route based on the increase in the rate of oxidation by several orders of magnitude compared to the rates of oxidation of the two substrates taken alone. Further their studies were supported by the presence of free radicals and stiochiometry of the reaction. In the mixture oxalic acid was oxidized by one electron transfer and iso-propanol by two electron transfer process. The end products are acetone and CO2. The brief mechanism where in a ter-molecular complex formed was supposed to give products in a slow step as shown in scheme 3.
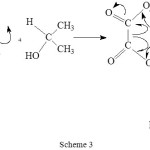 |
Scheme 3 Click here to View scheme |
Oxidations of A Substrate with three Functional Groups
Another interesting study by Roček on three electron oxidation is the intra-molecular cooxidation of a single substrate having three functional groups such as 2,7-dihydroxyheptanoic acid by chromic acid13. This was successfully discovered from the study of a series of dihydroxy carboxylic acids (HO(CH2)nCHOHCO2H). With n = 5 that is 2,7-dihydroxyheptanoic acid reacted completely in a different way compared to the other members of the series. 2,7-dihydroxyheptanoic acid is a substitute of two substrates one with two functional groups and the other with one functional group. The end products were corresponding aldehyde and alcohol. The transition state that formed is shown in scheme 4.
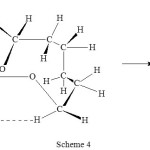 |
Scheme 4 Click here to View scheme |
Though the reaction sequence is not as efficient as the 2,7-dihydroxyheptanoic acid oxidation by Cr(VI), a similar study was also reported from our laboratory where in oxidation of glycerol which has three hydroxyl groups by chromic acid did take place by a three electron transfer route22. The end product of the reaction was glyoxal. The transition state is shown in scheme 5.
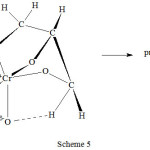 |
Scheme 5 Click here to View scheme |
Oxidation of Iso-Propanol by Mn(VII)
Kinetic study of oxidation of iso-propanol is more than a century old29. The reaction sequence involves the formation of permanganate ester which later gives the acetone as the product in a slow step. The reaction is shown in scheme 6.
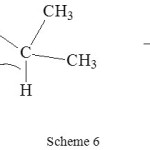 |
Scheme 6 Click here to View scheme |
Oxidation of Lactic Acid by Mn(VII)
Lactic acid is determined by the reaction of Mn(VII) in acid medium30. The products of oxidation were acetaldehyde and CO2. The reaction sequence is shown in scheme 7.
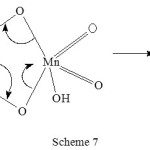 |
Scheme 7 Click here to View scheme |
Cooxidation by Mn(VII)
Our study was further extended to permanganate ion oxidations where in iso-propanol was oxidized by permanganate ion in presence of several bi-functional compounds by synchronous four electron transfer route23. The transition state taking lactic acid and iso-propanol as example is shown in scheme 8.
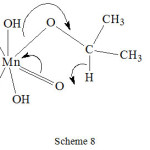 |
Scheme 8 Click here to View scheme |
Catalyzed and Un-Catalyzed Reactions
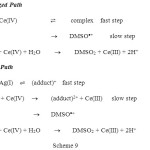
There were several thousands of catalyzed reactions reported in literature. Quoting them in the present article is beyond the scope of this article. But as an example to understand the concept of catalysis we present here a study involving Ag(I)catalyzed and un-catalyzed oxidation of dimethyl sulfoxide (DMSO) by Ce(IV) in nitric acid medium published from our labratory31. The product of oxidation was found to be dimethyl sulfone (DMSO2). The mechanism proposed for both the processes were shown in scheme 9.
 |
Scheme 9 Click here to View scheme |
In these reactions there is no change in concentration of Ag(I) at the end of the reaction which was confirmed by chromate titration.
Only Catalyzed Reactions
There were some instances in literature those are only catalyzed reactions. In the absence of the catalyst even under boiling conditions there was no reaction. One of such reactions was kinetics of Ag(I) catalyzed oxidative decarboxylation of some organic acids by Ce(IV) in H2SO4 medium32. The reaction sequence taking acetic acid (HA) as an example is shown in scheme 10.
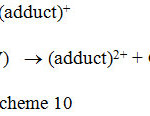 |
Scheme 10 Click here to View scheme |
The rest of the steps are similar to the steps 6 and 7 of the scheme 7.
Conclusions
From the above examples it was very clear that the process of cooxidation is different from catalysis. The essential condition for cooxidation to occur is one of the two substrates having two functional groups must be a good chelating agent with the metal ion oxidant. And in the catalytic reaction a catalyst in micro molar (mM) quantities must be able to catalyze the reaction with lower activation energies compared to the un-catalyzed reaction.
References
- Co-oxidation of isopropyl alcohol and oxalic acid by chromic acid: a one-step three electron oxidation. Fariza Hasan and Jan Roček, J. Am. Chem. Soc., 94, 3181 (1972)
CrossRef - Three-electron oxidations. II. The chromium (VI) oxidation of oxalic acid. Fariza Hasan and Jan Roček. J. Am. Chem. Soc., 94 9073 (1972)
CrossRef - Three-electron oxidations. III. The chromium (V) oxidation step. Fariza Hasan and Jan Roček. J. Am. Chem. Soc., 94, 8946 (1972)
CrossRef - Three-electron oxidations. IV. The chromic acid cooxidation of tertiary hydroxy acids and alcohols. Fariza Hasan and Jan Roček, J. Am. Chem. Soc., 95, 5421 (1973)
CrossRef - Three-electron oxidation. V. The rapid reaction of chromic acid with two component substrate systems. Fariza Hasan and Jan Roček. J. Org. Chem., 38, 3812 (1973)
CrossRef - Three-electron oxidation. VI. Chromic acid cooxidation of cyclobutanol and oxalic acid. The chromium (V) oxidation of cyclobutanol. Fariza Hasan and Jan Roček, J. Am. Chem. Soc., 96, 534 (1974)
CrossRef - Three-electron oxidations. VII. The pre-steady-state phase of the chromic acid oxidation of oxalic acid. Fariza Hasan and Jan Roček, J. Org. Chem., 39, 2612 (1974)
CrossRef - Three-electron oxidations. VIII. Direct evidence for the synchronous character of three-electron oxidations. F. Hasan and J. Roček. J. Am. Chem. Soc., 96, 6802 (1974). 70.
- Three-electron oxidations. IX. Chromic acid oxidation of glycolic acid. Fariza Hasan and Jan Roček. J. Am. Chem. Soc., 97, 1444 (1975). 71.
- Three-electron oxidations. X. Cooxidation of isopropyl alcohol and glycolic acid. F. Hasan and J. Roček. J. Am. Chem. Soc., 97, 3762 (1975)
CrossRef - Three-electron oxidations. XI. Chromium(V) oxidation of alcohols. Fariza Hasan and Jan Roček. J. Am. Chem. Soc., 98, 6574 (1976)
CrossRef - Three-electron oxidations. XII. Chromium(V) formation in the chromic acid oxidation of 2-hydroxy-2-methylbutyric acid. Miroslav Krumpolc and Jan Roček. J. Am. Chem. Soc., 99, 137 (1977)
CrossRef - Three-election oxidations. XIII. Intramolecular cooxidation of 2,7-dihydroxyheptanoic acid. Structure of the transition state in the chromium(VI) oxidation of alcohols. K.G. Srinivasan and J. Roček. J. Am. Chem. Soc., 100, 2789 (1978)
CrossRef - Three-electron oxidations. XIV. Carbon-13 isotope effect in the three-electron cooxidation of isopropyl alcohol and oxalic acid. S. Ramesh, J. Roček, and D. A. Schoeller. J. Phys. Chem., 82, 2751 (1978).
CrossRef - Three-electron oxidations. XV. Chromic acid oxidation of mandelic acid. Dominic Ip and Jan Roček. J. Org. Chem., 44, 312 (1979).
CrossRef - Three-electron oxidations. XVI. Chromic acid oxidation of mandelic acid. Dominic Ip and Jan Roček. J. Am. Chem. Soc., 101, 6311 (1979).
CrossRef - Three-electron oxidations. XVII. The chromium(VI) and chromium(V) steps in the chromic acid cooxidation of 2-hydroxy-2-methylbutyric acid and 2-propanol. S. N. Manapatro, M. Krumpolc and J. Roček, J. Am. Chem. Soc., 102, 3799 (1980).
CrossRef - Three-electron oxidations. XVIII. Carbon-13 and deuterium isotope effects in the cooxidation of 2-hydroxy-2-methylbutyric acid and 2-propanol. evidence for a two-step mechanism. S. Ramesh, S. N. Manapatro, J. H. Lin and J. Roček, J. Am. Chem. Soc. 103, 5172 (1981)
CrossRef - Kinetics and mechanism of oxidation of some aliphatic esters by chromic acid in the presence and absence of oxalic acid in acetic acid-water medium. P. Musala Reddy, V. Jagannadham, B. Sethuram and T. Navaneeth Rao, Indian J. Chem., Sect. A (1982) 21A, 483
- Co-oxidation of isopropanol and lactic acid in the presence of chromic acid: a case of three-electron oxidation study. V. Jagannadham, M. Anand Rao, B. Sethuram and T. Navaneeth Rao. Oxid. Commun., (1986/1985) 8, 31
- Three-electron oxidation: chromic acid oxidation of a mixed substrate system of an alcohol and aldehyde. P. Musala Reddy, V. Jagannadham, B. Sethuram and T. Navaneeth Rao, React. Kinet. Catal. Lett. (1982) 19, 243
CrossRef - Kinetics and mechanism of oxidation of glycerol by chromic acid in sulfuric acid medium. P. Musala Reddy, V. Jagannadham, B. Sethuram and T. Navaneeth Rao, Indian J. Chem., Sect. A (1982) 21A, 608
- A rapid reaction of Mn(VII) with two component substrate systems containing 2-propanol and some bi-functional compounds: a kinetic study. M. Anand Rao, B. Sethuram, T. Navaneeth Rao, and V. Jagannadham, Oxid. Commun. (1986) 9, 247
- The oxidation of organic substances by compounds of tervalent manganese: Oxidation of mandelic acid, ethylene glycol, glycerol and d-mannitol by the pyrophosphate complex of manganese(III) and by manganese(III) sulphate, J. Barek. .A. Berka. I. Procházková. Talanta, 1974, 21, 157
CrossRef - 7 things one may not know about catalysis, Louise Lerner, Argonne National Laboratory (2011), and also see the links: http://www.jagranjosh.com/general-knowledge/what-is-catalysis-1457685546-1, and https://www.google.co.in/?gfe_ rd=cr&ei=dAziVISUFsPM8geU7oDoCA&gws_rd=ssl#q=what+is+catalysis
- Oxidations with chromium(VI) oxide. VII. The mechanism of oxidation of secondary alcohols, J. Roček and J. Krupička, Collect. Czech. Chem. Commun. 1958, 23, 2068 (b) Chromic acid oxidation of iso-propyl alcohol, Kenneth B. Wiberg, and Hans Schaefer, J. Am. Chem. Soc., 1969, 91 , 933–936.
- Chromic acid oxidation of oxalic acid: kinetic investigation of the uncatalysed oxidation of oxalic acid by chromic acid, G.V. Bakore and C. L. Jain, Journal of Inorganic and Nuclear Chemistry, 1969, 31 Pages 805-810.
CrossRef - The chromic acid oxidation of oxalic acid: Evidence of chromium(IV) oxidation, F. Hasan and J. Roček, Tetrahedron, 1974, 30, Pages 21-24.
CrossRef - The oxidation of isopropyl alcohol with potassium permanganate, William Lloyd Evans, Lily Bell Sefton, J. Am. Chem. Soc., 1922, 44 (10), pp 2271–2276.
CrossRef - Theodore E. Friedemann and Arthur I. Kendall, The determination of lactic acid, J. Biol. Chem. 1929, 82, 23-43.
- Ag(I) catalyzed and un-catalyzed oxidation of dimethyl sulfoxide by Ce(IV) in nitric acid medium: A kinetic study, S. Venkateswar Rao and V. Jagannadham, React. Kinet., Catal., Lett., 1985, 27, 239.
CrossRef - Kinetics of Ag(I) catalyzed oxidative decarboxylation of some organic acids by Ce4+ in H2SO4 medium, M. Adinarayana, B. Sethuram and T. Navaneeth Rao, Current Science, Vol. 44, 1975, pp. 581-583.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









