Biological Enrichment of Chromite ore on Alkaline Roasting using Seidlitzia Rosmarinus Ash
Sedighe Sadat Marjani , Mohammad Hakimi and Hassan Ali Hossaini
, Mohammad Hakimi and Hassan Ali Hossaini
Department of Chemistry, Payam-e Noor University of Mashhad , Mashhad, Iran.
Corresponding Author E-mail : marjanisediqe@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340162
The Eco-friendly methods have become a promising synthetic strategy in science and technology in recent years. The current study describes alkaline roasting of chromite ore using Seidlitzia Rosmarinus (S. Rosmarinus) ash and water leaching of resulting cake. The Formation of Na2CrO4 was confirmed by Existing of ligand to metal charge transfer (LMCT) band in the UV-visible spectrum in 270 and 370 nm. Rhombohedral structure of the product was investigated using the powder X-ray diffraction (PXRD). The X-ray Fluorescence Spectroscopy (XRF) shows Increasing in chromium percent from 44.94 to 65.07 and existing elementals as sodium, potassium, calcium and magnesium in S. Rosmarinus ash that can act as alkaline assistance in alkaline roasting.
KEYWORDS:Biological; Enrichment; Chromite; Seidlitzia Rosmarinus
Download this article as:| Copy the following to cite this article: Marjani S. S, Hakimi M, Hossaini H. A. Biological Enrichment of Chromite ore on Alkaline Roasting using Seidlitzia Rosmarinus Ash. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Marjani S. S, Hakimi M, Hossaini H. A. Biological Enrichment of Chromite ore on Alkaline Roasting using Seidlitzia Rosmarinus Ash. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=42736 |
Introduction
Chromium and its byproducts have broad applications as stainless steel production, chromic acid plating, corrosion control and etc.1-3 chromium is present in many minerals, occasionally combined with iron oxides and other transition metal oxides such as manganese, titanium, vanadium, niobium and etc. 4
Chromite ore belongs to the spinel group with the general chemical formula of XY2O4 which X and Y represent divalent and trivalent metal ions .the natural mineral is usually represented by the general formula (Fe2+ ,Mg )(Cr, Al ,Fe3+)2O4 with sometimes small quantities of Magnesium , Titanium and Vanadium 5.
Production of sodium chromate from chromite ore is requisite reaction for producing other products 6. sodium chromate was manufactured by leaching of chromite ore using sodium hydroxide and sodium carbonate at temperatures 1000°C in an atmosphere of oxygen 7,8. A process such as soda- ash roasting 9, acid leaching 10, alkaline leaching 11,12, alkaline roasting using sodium hydroxide, potassium hydroxide and water leaching 13, have been developed for the processing of chromite ore in order to produce sodium chromate.
The conventional methods such as Pyro metallurgy or Hydrometallurgy, are not economic and ecofriendly. therefore Bioleaching of minerals is a suitable way for solves these problems. recently Bioleaching of ores using microorganisms was reported 14.
In this study, leaves and branches of S. Rosmarinus in oxidizing atmosphere was used as environmentally friendly and economical source for alkaline roasting of chromite and resulting solid cake was dissolved in distilled water to remove insoluble Iron (III) oxide and magnesium oxide from soluble chromates.
Experimental Methodology
The Concentrated chromite was obtained from a sabzan mine of faran, Kerman, Iran. The composition of concentrate sample used is given in table1. Concentrate was sieved into 200 mesh. S. Rosmarinus Leaves was collected from desert of bajestan, Khorasan, Iran.
The S. Rosmarinus leaves were cleaned with double distilled water, shade-dried and ground to powder and stored for further study.
3.3 g of chromite ore and 6.6 g of grinding plant was wearing up and uniformed. The mixture was transferred to crucible and putting up in 1100°C for 2h. Roasting mixture Chromite was cold and wear up in pounder and then mixed with water in 50 ml flask with fixed temperature 60°C in presence magnetic stirrer.
The UV–vis spectrum was recorded on a double beam spectrophotometer (Shimadzu, model UV-2550) from 200 to 500 nm. The solution was filtered, concentrated and dried in an oven for further analysis. Element Analysis of solid sample was confirmed by ED 2000 belong Oxford company of England X-ray Fluorescence (XRF) Spectrometer. for evaluation of the mineral structure of sodium chromate, X-ray powder diffraction using Cu-Kα radiation over an angle (2θ) range of 5 to 90°. With step: 0.04 with the support of X’ Pert software was used.
Results and Discussion
Characterization of chromite ore
The original mineral phase in chromite is FeCr2O4 .also existence of trace of titanium. nickel and manganese has been proven 15.
The elemental analysis results of chromium have shown in table 1. this result shows that the most percentage of elements respectively belong to chromium, silicon, iron and magnesium.
Table 1: Chemical composition of chromite was determinate by XRF
| Compound | Cr2O3 | SiO2 | Fe2O3 | MgO | Al2O3 | SO3 | P2O5 | MnO | CaO | TiO2 | NiO |
| Percentage | 44.94 | 20.47 | 18.89 | 10.06 | 2.91 | 0.56 | 0.55 | 0.45 | 0.22 | 0.16 | 0.16 |
Characterization of S. Rosmarinus ash
Chemical compositions of roasted plant in 1100°C determinate by XRF and were shown in table 2. this result shows the existence of elements such as sodium, potassium, calcium, magnesium and Strontium that confirmed alkaline assistance role of S. Rosmarinus ash.
Table 2: Chemical composition of S. Rosmarinus was determined by XRF
| Compound | CaO | SiO2 | K2O | Na2O | MgO | SO3 | Cl | Fe2O3 | Al2O3 | SrO | TiO2 | MnO | other |
| Percentage | 31.63 | 18.05 | 11.32 | 8.49 | 7.67 | 7.23 | 4.6 | 4.97 | 3.34 | 0.71 | 0.54 | 0.54 | 0.48 |
Roasting of Chromite using S. Rosmarinus ash and leaching with water
The comparison, chemical composition of chromium, S. Rosmarinus and soluble chromates in table 1,2 and 3, show a large percentage of chromium has been belonging to SiO2, that destroyed during the process. deletion of Fe2O3 and decreasing in the percentage of magnesium show formation of insoluble compound as Fe2O3 and MgO. Low change in the percentage of potassium and sodium, and increase in the percentage of chromium, confirm the formation of soluble chromates as Na2CrO4 and K2CrO4.
Table 3: Chemical composition of soluble chromates produced by roasting using S. Rosmarinus and leaching with water
| Compound | Cr2O3 | SO3 | Na2 O | K2O | Cl | MgO | Al2O3 | CaO | SiO2 | MnO | Sb2O3 |
| Percentage | 65.07 | 13.66 | 7.52 | 6.18 | 2.83 | 1.87 | 1.26 | 0.42 | 0.38 | 0.23 | 0.18 |
The Comparison chemical composition of S. Rosmarinus ash and soluble chromates in table 2 and 3 show decrease in the percentage of elements as calcium, strontium and magnesium, that Proves formation of insoluble chromates include CaCrO4, MgCrO4 and SrCrO4. This changes justified by equation 1 to 7.
Meanwhile roasting of chromite, probable reaction was accrued that equation 1to 7 shows this process.
(M is Representative of alkali metals)
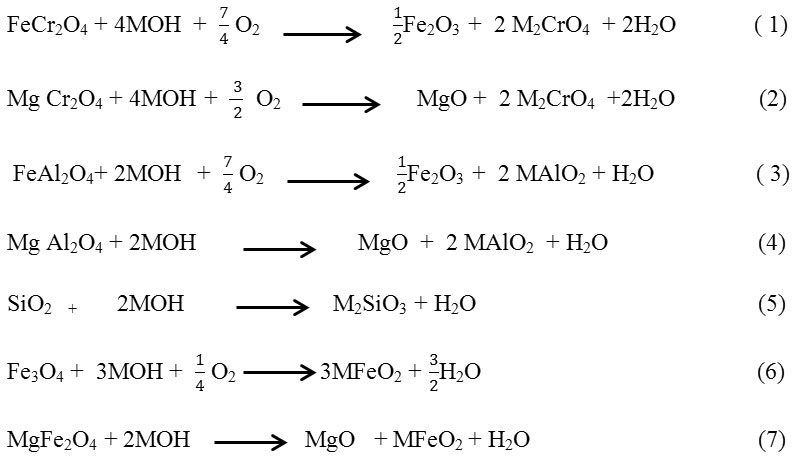
Equation 3-6 show possibility reaction of some of impurities as SiO2, FeAl2O4 and MgFe2O4 with MOH, but ∆G in equation 1 and 2 is more negative therefore the formation of Chromates is more likely 16.
Analysis of UV-Vis Spectroscopy
The UV–vis pattern of Na2CrO4 in Fig1 showed bands in 270 and 370nm mainly attributed to ligand -to -metal charge transfer (LMCT) band [17].
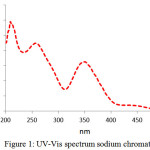 |
Figure 1: UV-Vis spectrum sodium chromate Click here to View figure |
XRD Studies
Fig 3 shows XRD Pattern of sodium chromate. Intense Peaks at the various angular position which indexed to JCPDS 22-1365 was attributed to Na2CrO4 with orthorhombic structure and network Parameters a=7. 1462 b=9.2635, c=5.864, existence a few impurities Na0.2K0.8CrO4 with monoclinic structure and network Parameters a=9. 939, b=5. 625, c=7. 163 were confirmed 18.
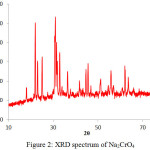 |
Figure 2: XRD spectrum of Na2CrO4 Click here to View figure |
Conclusion
In summary, the conversion of chromite ore to soluble chromates was achieved in two steps, alkali roasting by S. Rosmarinus ash and water leaching. XRF studies showed increases in the percentage of chromium from 44.94 to 65.07. Therefore S. Rosmarinus ash could act as Leach suppliers. UV-vis studies showed three picks due to charge transfers that confirmed formation of sodium chromate. The results obtained from XRD studies showed indicator picks due to orthorhombic structure of Na2CrO4.
Refrences
- Barnhart ,J.; J. soil contamination. 1997, 6, 561-568
- Dennis, J.K.; Such, T.E. J. Woodhead Publishing.1993,3,206-240
- Chen, G.; Wang, J.; Wang, X.; Zheng, S.L.; Du, H.; Zhang, Y.J. Hydrometallurgy. 2013,139, 46–53
CrossRef - Sanchez,S.S.; Makanyire, T.; Escudero,C.L.; Hara,Y.; Jha, A.J. Green. Chem . 2015, 17, 2059-2080
CrossRef - Prirenyatwa,S.; Escudero,C.L.; Sanchez,S.S.; Hara,Y.; Jha,A.J.Hydrometallurgy.2015, 4137, 14
- Gu, F.; Wills, B.A. J. Miner. Eng.1988,1, 235–240
CrossRef - Copson,R.L. J.Reinhold,1956,8,262-282
- Mark,H.F.; Othmer, D.F.; Overberger,C.G.; Seaborg,G.T.; Grayson, M.; J.Kirk-Othmer Encyclopedia of Chemical Technology, 1979,3, 82-120
- Tathavadkar ,V.D.; Jha, A.;Antony, M.J.Metal Mater Trans B,2001, 32,593-602
CrossRef - Gevice, A.; Topkayay , A.Y.; J.Miner .Eng, 2002,15 ,885-888
CrossRef - Xu, H. J. Miner Eng,2005, 18,527-535
CrossRef - Zhang,Y. J.Trans .Nonferrous. Metals. Soc. China. 2010,20,888-891
CrossRef - Amolpornwijit,W.; Meegoda,J.N.; Hu,Z.J. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2007, 11, 234–239
CrossRef - Ajim ,S.; Sutar,S.D. J.Int. Environ. Sci. Technol. 2015 , 5,14-21
- Ji, Z.J. Inorg. Chem. Ind. 2012, 44, 1–5
- Hwang,J.Y.; Seo,D.S. J. Electrochem. Society. 2010, 157,351-357
CrossRef - Woodward,D. Molecular Orbital Theory and Charge Transfer Excitations Chemistry, 2008 ,123 Spring
- Dettmer,A.; Nunes,K.; Gutterres.M.; Romeu,M.N.J. chem Eng.2010,160,8-12
CrossRef

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









