Lignin Degradation of Oil Palm Empty Fruit Bunches using TiO2 Photocatalyst as Antifungal of Fusarium Oxysporum
Maulidiyah Maulidiyah1, M. Natsir1, Fathmasari Fitrianingsih1, Zul Arham2, Dwiprayogo Wibowo1 and Muhammad Nurdin1
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Halu Oleo, Kendari 93292 - Southeast Sulawesi, Indonesia.
2Department of Mathematics and Natural Sciences, Faculty of Tarbiyah, Institute Agama Islam Negeri (IAIN) Kendari 93117 – Souteast Sulawesi, Indonesia.
Corresponding Author E-mail: mnurdin06@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330651
This study aims to obtain phenolic lignin derived compounds through the lignin degradation of oil palm empty fruit bunches (OPEFB) using TiO2 photocatalyst and its antifungal activity against Fusarium oxysporum. The lignin degradation of OPEFB begins with the extraction and isolation stage using the soxhletation method with the presence of sodium hydroxide as a catalyst. The success of this stage is indicated by Gas Chromatography (GC) measurement of 4-vinylguaiacol and Fourier Transform Infra Red (FTIR) spectroscopy measurement that indicated the appearance of seven specific peak of lignin occurring at wave numbers 3421 cm-1, 2929 cm-1, 1596 cm-1, 1508 cm-1, 1462 cm-1, 1041 cm-1, and 875 cm-1. This result is supported by the appearance of specific peaks of guaiacyl and syringyl groups at wave numbers 1328 cm-1 and 1220 cm-1. Based on the variation of the degradation time, the lignin degradation of OPEFB with a degradation time of 15 minutes showed optimum results in the production of lignin derived compounds. In addition, it also provides a strong inhibitory response to the growth of Fusarium oxysporum with a clear zone diameter 2.39 cm.
KEYWORDS:Lignin; OPEFB; TiO2 Photocatalyst; Fusarium Oxysporum; Clear Zone Diameter
Download this article as:| Copy the following to cite this article: Maulidiyah M, Natsir M, Fitrianingsih F, Arham Z, Wibowo D, Nurdin M. Lignin Degradation of Oil Palm Empty Fruit Bunches using TiO2 Photocatalyst as Antifungal of Fusarium Oxysporum. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Maulidiyah M, Natsir M, Fitrianingsih F, Arham Z, Wibowo D, Nurdin M. Lignin Degradation of Oil Palm Empty Fruit Bunches using TiO2 Photocatalyst as Antifungal of Fusarium Oxysporum. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40047 |
Introduction
In recent years, Indonesia has been reported as the world’s largest palm oil producing country with a production range of 85 to 90%, or about 37 million tones [i],[ii]. This production is expected to increase along with the increasing global population, consumption of food and cosmetic products that utilize palm oil [iii]. So that the production of palm oil will slowly increase the amount of waste, both liquid and solid [iv],[v].
OPEFB is a type of solid waste produced mostly from palm oil production. In 2016, the Agricultural Technology Assessment Center of Indonesia reported that about 60 million tons of OPEFB were produced in a year. This waste can pollute the air environment due to the odor generated and as a medium grows of pathogenic fungal that will interfere the growth of other plants around it. But on the other hand, it is potentially processed for various applications such as compost [vi], plywood adhesive [vii], biomass [viii], waste water purification [4], biofuel [ix], sugar production materials [x], and reinforcing fiber for polymer composite [xi]. This is due to OPEFB containing lignocellulose with cellulose (40-50%), hemicellulose (24-35%), and lignin (18-35%) [xii],[xiii].
In agriculture, lignin is widely used as an eco-friendly pesticide mixture [xiv],[xv]. Lignin degradation will produce phenolic derivatives and have high antifungal activity [[xvi],[xvii]]. So it is suitable to be developed as a material of pathogenic fungicides in agricultural crops.
Several methods such as enzymatic degradation and degradation of photocatalysis using TiO2 have been reported to be able to degrade lignin compounds [[xviii],[xix]]. Among these methods, lignin degradation using TiO2 photocatalyst has several advantages such as facilitating the formation of large reactive oxygen species, rapid degradation time, easy preparation, relatively inexpensive cost, and abundant raw material availability [xx],[xxi],[xxii].
Based on the above considerations, in this study the lignin of OPEFB was degraded in a UV reactor that it was developed previously [xxiii],[xxiv],[xxv] using TiO2 photocatalyst and tested its antifungal activity. Fusarium oxysporum is selected as a pathogenic fungal model to be tested. This fungal caused wilts, blights, bots, and cancerous diseases in the prime crop of agricultures.
Experimental Method
Sample Preparation
Samples of OPEFB (obtained from PT Damai Jaya Lestari, Konawe Utara-Southeast Sulawesi) are cleaned, dried in open space, and milled using a grinding machine. The resulting powder was then filtered using a 50 mesh sieve, dried in an oven at 60°C, and extracted with benzene : ethanol 96% at ratio 2 : 1 (v/v) for 6 hours. The obtained precipitate was dried and extracted again using water for 1 hour.
Lignin Isolation of OPEFB
Lignin isolation of OPEFB was carried out in the digester refers to the method reported by [i]. The OPEFB extract was performed into the digester with the addition of ethanol 96% : water at a ratio of 1 : 1 (v/v) and followed by the addition of soduim hydroxide 20%. The mixture was then cooked using a temperature of 170°C for 2 hours, filtered in a cold state, then the obtained filtrate was diluted using water at a ratio of 1 : 2 (v/v), and titrated using sulfuric acid 20%. The precipitate containing lignin was then centrifuged for 20 min at a rate of 4500 rpm and recrystallized.
Lignin Degradation of OPEFB
The degradation of lignin into its phenolic derivative was carried out using a TiO2 photocatalyst in a UV reactor. The degradation stage refers to the results of a study reported by [ii]. A total of 20 mL lignin solution of OPEFB with a concentration of 4 ppm was put into 5 vials and each added 0.5 g of TiO2. The lignin-TiO2 mixture was put into the UV reactor and degraded with variations of 5, 10, 15, 20 and 25 minutes.
Antifungal Activity of Fusarium oxysporum
A total of 10 μL cultures of Fusarium oxysporum were inserted in eppendorf tubes containing PDA media, then homogenized, poured into petri dishes, and allowed to solidify. Furthermore, 20 μL lignin of OPEFB was added to the tube and incubated for 3 days. The incubation results were observed and measured the clear zone formed. Fusarium oxysporum fungal rejuvenation was performed in a sterile PDA medium incubated for 5 days at room temperature.
Results and Discussion
Lignin Identification and Degradation of OPEFB
Lignin is an aromatic polymer formed by the dehydrogenative polymerization of the three cinnamyl alcohols (monolignols) p-coumaryl (4-hydroxycinnamyl), coniferyl (4-hydroxy-3-methoxycinnamyl) and sinapyl (4-hydroxy-3,5-dimethoxycinnamyl), which are the precursors of p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) lignin-units [iii].
Based on Martinez et al. explained that the main compounds identified by GC such as 4-vinylguaiacol and syringol [28]. The retention time in 26.63 minutes (Figure 1) has identified the 4-vinylguaiacol group which derived from guaiasyl group.
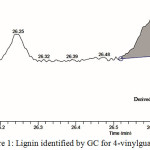 |
Figure 1: Lignin identified by GC for 4-vinylguaiacol Click here to View figure |
The fungsional group has identified by FTIR analysis to determine the success of the extraction and lignin isolation from OPEFB. Figure 2 shows the seven specific absorbing lignin peaks occurring at the wave number 3421 cm-1 for the -OH vibration, 2929 cm-1 for the vibration of C-H from the methyl, 1596 and 1508 cm-1 for the C=C vibration of the aromatic ring, 1462 cm-1 for asymmetric C-H vibration, 1041 cm-1 for the -OH vibration of aliphatic or ether, and 875 cm-1 for the C-H vibration of the aromatic group. In addition, there are also two absorption peaks at wave numbers 1328 cm-1 and 1220 cm-1 indicating the presence of a syringyl and guaiasyl rings for lignin [i]. The emergence of this specific peak indicates the success of our extraction and isolation processes in this work.
Lignin of OPEFB were degraded using anatase TiO2 by UV light irradiation at 365 nm wavelength range in a reactor. Anatase TiO2 has high photocatalytic properties that will result in excellent degradation activity [ii],[iii],[iv]. In addition, the use of anatase TiO2 refers to the principle of photocatalytic lignin degradation, where degradation occurs at low energy with a wavelength range between 300 nm and 400 nm [v],[vi]. In the wavelength range, it is reported as the optimum light absorption area of anatase TiO2 (Figure 3) [vii],[viii].
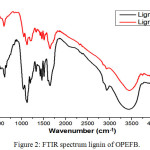 |
Figure 2: FTIR spectrum lignin of OPEFB. Click here to View figure |
The time variation of lignin degradation is an attempt to prevent lignin degradation completely. Anatase TiO2 can generally degrade organic molecules into H2O and CO2 [i],[ii]. Lignin degradation will produce 4-hichroxyphenyl, guaiacyl, and syringyl groups [28]. The groups are lignin derivatives which are known like p-cumaril alcohol, coniferil alcohol, and sinapil alcohol to have high antifungal properties (Figure 3).
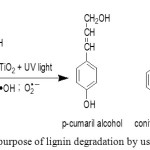 |
Figure 3: Mechanism purpose of lignin degradation by using TiO2 photocatalyst Click here to View figure |
Antifungal Activity
The antifungal activity test of lignin derivative compounds was performed using well diffusion method. This method is reported as an effective method of testing antifungal activity [i]. Figure 4A shows the results of antifungal activity test against Fusarium oxysporum. These results suggest that the variation of degradation time used in this work is capable of producing lignin derived compounds that can inhibit Fusarium oxysporum growth. Based on the measurement of the diameter of the clear zone (Figure 4), it is known that the lignin degradation for 15 minutes has a high inhibitory effect on the growth of Fusarium oxysporum with the clear zone diameter is 2.39 cm. This diameter is reported to have a strong growth barrier response for a microorganism [ii].
Other than that, Figure 4B also illustrates the effect of time on the lignin degradation of OPEFB using TiO2 photocatalyst. The optimum degradation time for lignin occurs in the 15 minutes. A degradation time span of 5 to 10 minutes indicates that there is still a considerable amount of lignin that has not been degraded, while in the span of 20 to 25 minutes indicates that lignin decomposes perfectly to produce molecules that have no phenolic properties.
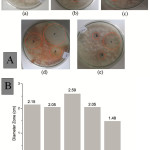 |
Figure 4: (A) Antifungal activity test of OPEFB lignin derivative against Fusarium oxysporum with degradation times:Click here to View figure |
(a) 5 minutes, (b) 10 minutes, (c) 15 minutes, (d) 20 minutes, and (e) 25 minutes. (B) Plot of clear zone diameter versus degradation times.
Lignin Degradation Mechanism of OPEFB with the Presence TiO2 Photocatalyst
Lignin degradation of OPEFB begin when the surface of TiO2 is exposed to UV light with a wavelength of 365 nm. Electrons in the ground state (low energy levels) will absorb the light so that it is excited at higher energy levels while leaving strong oxidizing holes (h+). The hole (h+) will oxidize the H2O molecule or the OH– ion to form a hydroxyl radical (OH•). In addition, the excited electron (e–) will reduce the O2 molecule to form a super oxide (O2•–) which will further facilitate the formation of H2O2 and OH• molecules [i],[ii],[iii]. These formed molecules are reactive oxygen species (ROS) that play a role in degrading the lignin of OPEFB. The lignin degradation mechanism of OPEFB with the presence of TiO2 photocatalyst is shown in Figure 5.
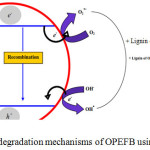 |
Figure 5: Lignin degradation mechanisms of OPEFB using TiO2 photocatalyst. Click here to View figure |
Conclusion
The success of lignin extraction and isolation process from OPEFB was characterized by the emergence of nine specific absorption peaks at wave numbers of 3421 cm-1, 2929 cm-1, 1596 cm-1, 1508 cm-1, 1462 cm-1, 1328 cm-1, 1220 cm-1, 1041 cm-1, and 875 cm-1. The use of TiO2 photocatalyst was able to degrade the lignin of OPEFB, where optimum degradation time occurred in the 15th minute. The antifungal activity test of lignin and derivative compounds carried out by well diffusion method that gave a strong inhibitory response to the growth of Fusarium oxysporum.
Acknowledgement
We acknowledge for financial support of the DRPM-Ministry of Research, Technology and Higher Education, the Republic of Indonesia.
References
- Maulidiyah; Nurdin, M.; Fatma, F.; Natsir, M.; Wibowo, D. Anal. Chem. Res., 2017, 12, 1-9.
CrossRef - Dahnum, D.; Tasum, S.O.; Triwahyuni, E.; Nurdin, M.; Abimanyu, H. Energy Procedia, 2015, 68, 107-116.
CrossRef - Triwahyuni, E.; Hariyanti, S.; Dahnum, D.; Nurdin, M.; Abimanyu, H. Procedia Chemistry, 2015, 16, 141-148.
CrossRef - Sari, A.A.; Kurniawan, H.H.; Nurdin, M.; Abimanyu, H. Energy Procedia, 2015, 68, 254-262.
CrossRef - Liew, W.L.; Kassim, M.A.; Muda, K.; Lo, S.K. ARPN J. Eng. & Appl. Sci., 2016, 11, 2400-2405.
- Siddiqui, Y.; Meon, S.; Mohd, R.I.; Rahmani, M.; Ali, A. Asian J. Microbiol. Biotechnol. & Environ. Sci., 2009, 11, 1-6.
- Risanto, L.; Hermiati, E.; Sudiyani, Y. Makara J. Technol., 2014, 18, 67-75.
CrossRef - Sudiyani, Y.; Styarini, D.; Triwahyuni, E.; Sudiyarmanto; Sembiring, K.C.; Aristiawan, Y.; Abimanyu, H.; Han, M.H. Energy Procedia, 2013, 32, 31-38.
CrossRef - Choi, W.I.; Park, J.Y.; Lee, J.P.; Oh, Y.K.; Park, Y.K.; Kim, J.S.; Park, J.M.; Kim, C.H.; Lee, J.S. Biotechnol. for Biofuels, 2013, 6, 1-8.
CrossRef - Zulkiple, N.; Maskat, M.Y.; Hassan, O. Procedia Chemistry, 2016, 18, 155-161.
CrossRef - Faizi, M.K.; Shahriman, A.B.; Majid, M.S.A.; Shamsul, B.M.T.; Ng Y.G.; Basah, S.N.; Cheng, E.M.; Afendi, M.; Zuradzman, M.R.; Wan, K.; anf Hazry, D. Matec Web of Conferences, 2017, 90, 1-9.
- Richana, N.; Winarti, C.; Hidayat, T.; Prastowo. Int. J. Chem. Engin. & Appl., 2015, 6, 422-426.
- Hayawin, Z.N.; Astimar, A.A.; Ibrahim, M.H.; Wan, H.; Khalil, H.P.S.A. J. Oil Palm Res., 2011, 23, 979-989.
- Sun, Y.; Ma, Y.; Fang, G.; Ren, S.; Fu, Y. BioResources, 2016, 11, 2361-2371.
- Perez, M.F.; Herrera, F.J.G.; Pradas, E.G. J. Hazardous Mater., 2011, 190, 794-801.
CrossRef - Yoshikawa, T.; Yagi, T.; Shinohara, S.; Fukunaga, Y.; Nakasaka, T.; Tago, T.; Masuda. Fuel Processing Technol., 2013, 108, 69–75.
CrossRef - Chen, F.; Longa; Xiaohua, L.; Yua, M.; Liua, Z.; Liua, L.; Shao, H. Industrial Crops and Products, 2013, 47, 339– 345.
CrossRef - Prado, R.; Xabier, E.; Jalel, L. Chemosphere, 2013, 91, 1355-1361.
CrossRef - Machado, A.E.H.; Furuyama, A.M.; Falone, S.Z.; Ruggiero, R.; Perez, D.S.; Castellan, A.; Chemosphere, 2000, 40, 115-24.
CrossRef - Maulidiyah; M. Nurdin; Wibowo, D.; Sani, A. Int. J. Pharma. Pharmaceu. Sci., 2015, 7, 141-146.
- Maulidiyah; Wibowo, D.; Hikmawati; Salamba, R.; Nurdin, M. Orient. J. Chem., 2015, 31, 2337-2342.
CrossRef - Nurdin, M.; Maulidiyah; Watoni, A.H.; Abdillah, N.; Wibowo, D. Int. J. ChemTech Res., 2016, 9, 483-491.
- Maulidiyah; Ritonga, H.; Salamba, R.; Wibowo, D.; Nurdin, M. Int. J. ChemTech Res., 2015, 8, 645-653.
- Nurdin, M.; Zaeni, A.; Rammang, E.T.; Maulidiyah, M.; Wibowo, D. Anal. Bioanal. Electrochem., 2017, 9, 480-494.
- Maulidiyah; Tribawono, D.S.; Wibowo, D.; Nurdin, M. Anal. & Bioanal. Electrochem., 2016, 8, 761-776.
- Kim, H.; Hill, M.K.; Friche, A.L. Tappi Journal, 1987, 70, 112-116.
- Nair, V.; Piyali, D.; Vinu, R. RSC Adv., 2016, 6, 18204-18216.
CrossRef - Martinez, A.T.; Camarero, S.; Gutierrez, A.; Bocchini, P.; Galletti, G.C. J. Anal. & Appl. Pyrolysis, 2001, 58–59, 401–411.
CrossRef - Watkins, D.; Nuruddin, Md; Mahesh, H.; Tcherbi-Narteh, A.; Jeelani, S. J. Mater. Res. & Technol., 2014, 4, 26-32.
CrossRef - Maulidiyah; Ritonga, H.; Faiqoh, C.E.; Wibowo, D.; Nurdin, M. Biosci. Biotechnol. Res. Asia, 2015, 12, 985-1989.
- Maulidiyah; Nurdin, M.; Widianingsih, E.; Azis, T.; Wibowo, D. ARPN J. Engin. & Appl. Sci., 2015, 10, 6250-6256.
- Arham, Z.; Nurdin, M.; Buchari, B. Int. J. ChemTech Res., 2016, 9, 113-120.
- Maulidiyah; Nurdin, M.; Erasmus, Wibowo, D.; Natsir, M.; Ritonga, H.; Watoni, A.H. Int. J. ChemTech Res., 2015, 8, 416-423.
- Nurdin, M.; Muzakkar, M.Z.; Maulidiyah, M.; Nurjannah, M.; Wibowo, D. J. Mater. & Environ. Sci., 2016, 7, 3334-3343.
- Nurdin, M.; Maulidiyah; Watoni, A.H.; Abdillah, N.; Wibowo, D. Int. J. ChemTech Res., 2016, 9, 483-491.
- Nurdin, M.; Zaeni, A.; Maulidiyah; Natsir, M.; Bampe, A.; Wibowo, D. Orient. J. Chem., 2016, 32, 2713-2721.
CrossRef - Maulidiyah; Azis, T.; Nurwahidah, A.T.; Wibowo, D.; Nurdin, M. Environ. Nanotechnol., Monit. & Manag., 2017, 8, 109-111.
- Maulidiyah, M.; Wibowo, D.; Herlin, H.; Andrini, M.L.; Ruslan; Nurdin, M. Asian J. Chem., 2017, 29, xx-xx.
- Magaldi, S.; Mata-Esayag, S.; Capriles, C.H.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Int. J. Infectious Diseases, 2004, 8, 39-45.
CrossRef - Handayany, G.N. J. Islam & Sci., 2015, 2, 347-388.
- Nurdin, M. Int. J. Pharma & Bio Sci., 2014, 5, 360-369.
- Ruslan; Mirzan, M.; Nurdin, M.; Wahab, A.W. Int. J. Appl. Chem., 2016, 12, 399-409.
- Nurdin, M.; Ramadhan, L.O.A.N.; Darmawati; Maulidiyah; Wibowo, D. J. Coat. & Technol. Res., 2017, DOI: 10.1007/s11998-017-9976-8.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









