In Silico Study, Synthesis, and Cytotoxic Activity of Esterification of Eugenol and Gallic Acid Against HT-29 Cell Line
Fadilah1, Arry Yanuar2 , Ade Arsianti1, Retnosari Andrajati2 and Rafika Indah Paramita1
, Ade Arsianti1, Retnosari Andrajati2 and Rafika Indah Paramita1
1Department of Medical Chemistry, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia.
2Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia.
Corresponding Author E-mail: arry.yanuar@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330638
Eugenol and gallic acid have cytotoxic effects in HT-29 colon cancer cells. In this study, we examined cytotoxic effect of esterification of eugenol and gallic acid. Before synthesized process, in silico docking was conduct to eugenol, gallic acid and its esterification products against Bcl-2 protein. After in silico docking, Eugenol and gallic acid were allowed to react by Mitsunobu Reaction to obtain compound (4-5). Proton and carbon NMR spectroscopy, also mass spectra (GCMS) were used to characterize of the structure from products. The products from esterification were tested by cytotoxic activity and showed by IC50 with navitoclax as positive control. In silico docking result showed compound (5) had the lowest Gibb’s Energy. Compound 4 and 5 were successfully synthesized and characterized by spectrometer 1H-NMR, 13C-NMR, and mass spectrometry. In silico docking result related to cytotoxic assay against HT-29 cell line. By replacing hydroxyl group of gallic acid with methoxy groups had increased the lipophilicity and the cytotoxicity. The greatest inhibitor against HT-29 cell line was compounds (5) with IC50 values of 22.81 µg/ml. The synthesized esterification product, compound (5), had greater cytotoxic activity than eugenol and gallic acid against colon cancer cell line, HT-29. Thus, the esterification products should be considered as a promising candidate for cancer colon drug.
KEYWORDS:Cytotoxic; Eugenol; Gallic Acid; Esterification; Mitsunobu Reaction; HT-29 cell line
Download this article as:| Copy the following to cite this article: Fadilah F, Yanuar A, Arsianti A, Andrajati R, Paramita R. I. In Silico Study, Synthesis, and Cytotoxic Activity of Esterification of Eugenol and Gallic Acid Against HT-29 Cell Line. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Fadilah F, Yanuar A, Arsianti A, Andrajati R, Paramita R. I. In Silico Study, Synthesis, and Cytotoxic Activity of Esterification of Eugenol and Gallic Acid Against HT-29 Cell Line. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=39753 |
Introduction
Cancer is one of the major concerns disease around the world, it is cause of the hignest mortality rate in the world.1 Colorectal cancer (CA) ranks third in men with 746,000 cases of patients, 10% of CA, whereas in women it ranks second in 614,000 cases, 9.2% of CA.2 It is predicted that about 90% of the colon cancer happend after the age of 50 (American cancer).1
Nowadays, a lot of research in synthesis to find new potential anticancer drugs. Currently, many potential anticancer drugs have been created, but some of these drugs still do not meet due to several factors.3 Therefore, research for selective and new anticancer drugs are needed, as current anticancer drugs have side effects and many are resistant.4
Eugenol is an active compound of cloves (Syzgium aromaticum L.), the compound is based on research Majeed et al, 2014 has activities as anticancer.5 Jaganathan et al, 2011 conducted that eugenol can suppress the proliferation of colon cancer cells with capturing cells at phase sub-G1, in addition to DNA fragmentation due to increased ROS levels and apoptosis.6 Eugenol is a phenolic compounds that have colorectal anticancer properties by inducing apoptosis. While other studies mention eugenol in HT-29 colon cancer cells modulate expression growth of COX-2 (cyclooxygenase-2).7
Gallic acids (3,4,5-trihydroxyl-benzoate) are aromatic benzoate compounds at present in parts of some plants. These compounds have biological activities such as antibacterial, antiviral, antiinflammatory, and also antitumor.4 Jaikumar and Jasmine 2016 was condacted that galloyl derivatives with different substituents have affect cytotoxic activity, such as digalloylresveratrol against in human colon cancer cells HT -29 and human leukemia cells HL-60 with induce apoptosis. While the right substituents in the galloyl group positions have a stronger effect.8
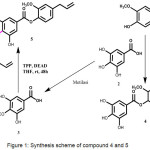 |
Figure 1: Synthesis scheme of compound 4 and 5 Click here to View figure |
Apoptosis (programmed cell death) is highly conserved and regulated process, which is the primary mechanism for the removal of aged, damaged, and unnecessary cells. Its deregulation can lead to cancer development and poor response to conventional chemotherapy. Cellular proteins of Bcl-2 family are a crucial and fundamental component of apoptosis and include the members of antiapoptotic and proapoptotic, the membrane permeabilization.9 the protein of Bcl-2 members are located in mitochondrial membrane. There are an intrinsic pathway of apoptotic regulator that acts by controlling the release of cytochrome c and other mitochondrial intermembrane proteins into the cytosol.10
With an increasing number of known experimental target molecules, computational methods have been used to significantly supplement and expedite the drug designing process. In silico analysis is the most straight forward approach to discover and predict novel lead molecules in less time and cost. Autodock is good molecular docking software which helps in predicting the binding site of ligand-protein interaction. In this research, we will test the activity of eugenol and its esterification results against HT-29 colon cancer cells. It is expected from this study that esterification products can be used as candidates for colorectal anticancer agents.
Experimental
Drug Likeliness Evaluation
Lipinski rule of five were used to evaluate under Marvin View Properties Tools. Lipinski rule of five were used to describe the pharmacokinetics properties of drugs in the body. This rule provides a condition for the candidate of the compound to be used as a drug.
Preparation of Protein
Structure the protein of Bcl-2 (PDB id: 4LXD) obtained from Protein Data Bank (http://www.pdb.org/). The macromolecule was prepared to calculate the binding energy using AutoDock tools. Water and non-standard residue were removed from protein. Hydrogen and Gasteiger partial charges were further added to the carbon that held the hydrogen. The binding pocket of the protein was determined by grid based approach using default parameters. The grid box was made using docking grids of 50*50*50 points in ordinate (x, y, z); (26.902, 30.781, 8.365) spanning cavity of the protein and spacing point of the grid was 0.380 Å. The grid box includes the active region of the protein that becomes the point in the binding of the ligand.
Preparation of Ligand
The structure of compound was made in 2D using Marvin Sketch 15.1.19 software and saved in 3D structure in .pdb format. The ligands then being optimized using AutoDock tools to fix the charge, added the hydrogen and minimizing energy. The 3D structure then saved in .pdbqt format.
Molecular Docking and their Interaction Studies
Molecular Docking of designed compounds was carried out with Lamarckian genetic algorithm default in Autodock4.2 tools. We selected Autodock4.2 tool for the purpose of molecular docking because AutoDock is an effective tool that could predict quickly and accurately bound conformations and binding energies of ligands with macromolecular targets.
The success rate in binding of protein-ligand complexes is known from the docking program validation results. The unit used for the ligand-complex binding validation is RMSD. The RMSD between the lowest energy docked Bcl-2 ligand pose and the Bcl-2 ligand native pose was evaluated using PyMol 1.7.4.5.
Docking interactions were clustered to determine the Gibbs energy (ΔG) and low of ΔG that conformation energy of the best-docked value. An estimated inhibition concentration (Ki) was reported to determine the binding energy which produced from the docking has different conformations on each compound, correlation with binding energy value.11
Synthesis Reaction
Chemicals and reagents that used in this research were purchased from Merck and Sigma Aldrich. Target synthesis (4-5) were synthesized using esterification reaction of eugenol (1) with gallic acid (2) or 3-methoxy gallic acid (3). The reaction was done using TPP, DEAD and THF as a solvent at room temperature (Figure 1). The synthesized compounds showed in good to excellent yields.
1H NMR (proton NMR) was identified using JEOL JNM-ECP (500 Hz) in CDCl3 as solvent. 13C NMR (carbon NMR) was identified using JEOL JNM-ECP (125 Hz) in CDCl3 as solvent. The measurement results are known from the difference of the chemical shifts which expressed in ppm and coupling constants are measured in Hertz (Hz). The reactions were checked with TLC using aluminum silica gel 60 F254 2×5 cm (Merck). Ultra-violet light (254 nm) and iodine vapours were used to visualized the chromatograms. Mass spectra (MS) were recorded on Shimadzu GCMS QP-5000.
Biological Activity with in vitro test
The inhibitory effect of synthesized compounds against colon cancer cell HT-29, was identified with MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. First, cells were cultured in 96 well plates with a total of 5000 cells/wells and seeded for 24 hours. The next step of the media was removed and added 3.125-100 ppm of eugenol, gallic acid and esterified product, respectively, then incubated for 24 hours. The next step was added to each well of 20 μM of MTT solution and incubated for 4 hours at 37°C. Supernatant and Crystals MTT was dissolved with dimethyl sulfoxide (DMSO) 100 μl. Last result observed absorbance with elisa reader at wavelength 570 nm The percentage of viable cells was plotted versus the concentration of the test compound. The concentration by which to mediate 50% cytotoxicity (IC50) was determined by linear regression analysis.
Result and Discussion
In Silico Study
The drug-likeness of compounds can be predicted by Lipinski’s Rules of Five which refers to the similarity of compounds to oral drugs. The molecular docking process predicts ligand confirmation and orientation on an active site that is the target of the ligand and has an important role in drug design.13 According Lipinski, the absorption and permeability of compounds with molecular weight above 500 possibilities is worse. While log P is not more than 5, where log P expresses solubility of the compound in octanol/water, for the amount of H acceptor no more than 10 and H donor no more than 5.12 In this study, all the compounds are satisfying Lipinski’s rules and showed in table 1.
Table 1: Lipinski properties of the compounds
| No | MW (g/mol) | HBD | HBA | LogP | Lipinski |
| 1 | 164.20 |
1 |
2 |
2.61 |
Y |
| 2 | 170.12 |
4 |
5 |
0.72 |
Y |
| 3 | 184.15 |
3 |
5 |
0.87 |
Y |
| 4 | 316.31 |
3 |
6 |
2.66 |
Y |
| 5 | 344.36 |
2 |
6 |
3.20 |
Y |
Docking studies were performed to evaluate the effect of ligands on the macromolecules Bcl-2. The result of docking simulation of compounds can be seen in table 2. Indicator from docking simulations can be seen by comparing the value of the Gibbs energy (ΔG) and inhibition constant. Gibbs energy (ΔG) showed the stability interaction between ligand and Bcl-2 residues, whereas inhibition concentration (Ki) was determined the binding energies of different docking conformation. Interaction between ligand and protein showed in figure 2.
Table 2: Molecular docking interaction with Bcl-2
|
Compound |
ΔG (Kcal/Mol) |
|
1 |
-4.76 |
|
2 |
-4.07 |
|
3 |
-4.37 |
|
4 |
-6.59 |
|
5 |
-6.90 |
|
Navitoclax |
-10.26 |
As for compound 4 (fig. 2a), the presence of hydrogen bonds around macromolecules in the binding site area owned by ligand is in Leu134, Arg143 and Gln133 with distance 2.9 Å, 3.2 Å, and 2.8 Å, respectively. Compound 5 (fig. 2b) showed an interaction on the binding site residues of Arg143, Arg143 and Gln133 with the distances of 3.6 Å, 3.1 Å, and 3.3 Å, respectively. Navitoclax (fig. 2c) as a positive control has hydrogen bond on the residues Asn140, Gly142, Arg143, Tyr190 which is 3.2, 2.6, 3.0, and 3.3 Å. The five compounds exhibiting strong interaction results compared with the positive control compounds. This indicates that the screening results of the five compounds designed can be used as candidates for synthesis as an inhibitor compound Bcl-2 apoptotic regulator inhibitor. Important interactions are shown all in the domain BH3 region.
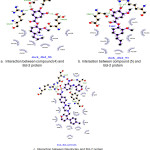 |
Figure 2: Interaction between compounds and protein Click here to View figure |
Synthesis of the Desired Compounds (4-5)
Synthesis of the target compounds (4-5) were started from Mitsunobu Reaction that changed carboxylic site bounded in aromatic rings to esters site. This reaction using triphenylphosphate (TPP), azodicarboxylate diisopropyl (diad), and alcohol that had stereochemistry invertion. Target compound (5) use monomethoxy gallic acid (3) as starting material and target compound (4) use gallic acid (1) as starting material. We had to synthesize (3) from gallic acid by methylation reaction using methyl iodide / K2CO3, and give monomethoxymethyl gallate as a product. Then we had to hydrolize the previous product, using aqueous Lithium hydroxide, and give monomethoxy gallic acid as aproduct. The structures of product synthesized were identified by 1H NMR, 13C NMR, and mass spectrometry. The spectroscopic data of title compounds (3-5) are presented as follows:
Compound (3)
Compound 3 was obtained and purification by column chromatography on silica gel (chloroform) purple powder; yield 35% ; 1H-NMR (500 MHz, CDCl3): δ 7.02 (s, 2H), 3.78 (s, 3H); 13C-NMR (125 MHz, CDCl3): δ 165.4, 152.0, 145.2, 125.3, 109.6, 60.8; MS (m/z): 184.2 (M+) / C8H8O5.
Compound (4)
This compounds was obtained and purification by column chromatography on silica gel (chloroform) and identification by TLC (MeOH/CHCl3 = 4:1) with Rf= 0.80; brown liquids; yields 38%; 1H-NMR (500 MHz, CDCl3): δ 7.41 – 7.39 (d, J=6.0 Hz, 1H), 7.02 (s, 2H), 6.82 – 6.80 (m, 2H), 6.00 – 5.98 (m, 2H), 5.12 – 5.10 (m, 2H), 3.83(s, 3H), 3.41 – 3.39 (d, J=6,0 Hz, 2H); 13C-NMR(125 MHz, CDCl3): δ 165.4, 151.2, 145.2, 140.2, 137.7, 136.0, 135.4, 125.3, 122.4, 121.7, 115.9, 113.5, 109.6, 58.0, 40.0; MS (m/z): 316.0 (M+) / C17H16O6.
Compound (5)
This compounds was obtained and purification by column chromatography on silica gel (EtOAc/hexanes = 1:1) with Rf= 0.65; brown yellow liquids; yields 41%; 1H-NMR (500 MHz, CDCl3): δ 7.39 – 7.37 (d, J=6.0 Hz, 1H), 7.02 (s, 2H), 6.80 – 6.78 (m, 2H), 5.98 – 5.96 (m, 2H), 5.10 – 5.08 (m, 2H), 3.81 (s, 3H), 3.78 (s, 3H), 3.40 – 3.38 (d, J=6,0 Hz, 2H); 13C-NMR(125 MHz, CDCl3): δ 165.4, 152.0, 151.2, 145.2, 137.7, 136.0, 135.4, 125.3, 122.4, 121.7, 115.9, 113.5, 109.6, 60.8, 58.0, 40.0; MS (m/z): 330.1 (M+) / C18H18O6.
Two compounds of esterification product of eugenol and gallic acid (4-5) were synthesized successfully. The chemical structures of these compounds were identified by 1H NMR, 13C NMR, and mass spectrometry. 1H-NMR spectrum confirmed the required number of H-atoms of the compound (5). The presence of methoxy group in aromatic ring of compound (5) was supported by the appearance of sharp singlet at δ 3.81 (ppm) in the 1H-NMR spectrum. 13C-NMR spectrum confirmed the addition of carbon at δ (ppm) 60.8 that showed addition of methoxy group in compound (5). Furthermore, the molecular weight of compound (5) was found to be appropriate with the target structures.
Biological activity with in vitro test
Activity test is done by calorimetry method using MTT which indirectly can determine the number of living cell with in vitro.HT-29 cells were added with variation dose of eugenol, gallic acid and products (3.125–100 µg/ml) for 24 h. Compound 5 showed that compound have strong antiproliferative activity against HT-29 cells (IC50 = 22.81 ± 1.65 µg/ml) (Table 3).
Table 3: IC50 value of the compound
|
Compound |
IC50 (µg/ml) |
|
1 |
28.31 ± 1.14 |
|
2 |
34.19 ± 0.92 |
|
3 |
32.25 ± 1.81 |
|
4 |
25.76 ± 1.58 |
|
5 |
22.81 ± 1.65 |
|
Navitoclax |
0.65 ± 0.88 |
IC50 is the 50% half maximal inhibitory activity in µg/mL, expressed in mean value (n=3) ± SD. SD: Standard deviation
After completion of the synthesis, cytotoxic activity of compound (1-5) were evaluated against HT-29 cell line, cytotoxic activity represented by IC50. The smaller IC50 value, the higher cytotoxic activity. Cytotoxic activity of compound (1-5) and navitoclax as positive control are showed in Table 3. Eugenol, gallic acid, and its esterification products have IC50 value around 30 µg/mL which are assigned as moderately toxic compounds, whereas compound (5) is the most toxic compound. This result suggesting that replacing hydroxyl group of gallic acid with methoxy groups will increase the lipophilicity and will increase the cytotoxicity. This fact suggested hydroxyl group and monomethoxy group on the aromatic ring are very important for selectivity and cytotoxic activity. Furthermore, the cytotoxic activity of the compound had same correlation with in silico docking result.
Conclusion
Esterification products of eugenol and gallic acid have been synthesized successfully. The target compound exhibit a greater cytotoxic activity than eugenol and gallic acid against colon cancer cell line HT-29. Thus, the esterification products should be considered as a promising candidate for cancer colon drug.
Acknowledgements
We thank to Universitas Indonesia for the Publikasi Terindeks Internasional Untuk Tugas Akhir Mahasiswa UI (PITTA) research grant.
References
- Devi, Y. P. Int. J. Res. Applied. 2014, 2, 269–272.
- Ferlay, J. et al. Int. J. cancer. 2015, 136.
- Shukla, S., Srivastava, R. S., Shrivastava, S. K., Sodhi, A. & Kumar, P. Asian Pac. J. Trop. Biomed. 2012, 5, 1040–46.
CrossRef - Jaikumar, B. & Jasmine, R. Int. J. PharmTech Res. 2016, 9, 333–365.
- Majeed, H., Antoniou, J. & Fang, Z. Asian Pac. J. Cancer Prev. 2014, 15 (21), 9159–9164.
CrossRef - Jaganathan, S. K., Mazumdar, A., Mondhe, D. & Mandal, M. Cell Bio. Int. 2011, 35, 607–615.
- Suk, S. et al. Life Sci. 2003, 73, 337–348.
CrossRef - Carvalho, J. E. De, Vieira, C., Samara, A. & Formagio, N. Molecules. 2015, 205360–5373.
- Hsu, J.-D. et al. J. Agric. Food Chem. 2011, 59, 1996–2003.
CrossRef - Pollio, A. et al. Molecules. 2016, 21, 1–23.
CrossRef - Shipra G, Gauri M, Chandra PM, Kishore SP. Protein & Peptide Letters. 2012, 19,1302–17.
CrossRef - Morris, G. M. et al. J. Comput. Chem. 1998, 19, 1639–1662.
CrossRef - Simon JP, Shallauddin KB, Ramalingam G, Gunaseelan D, Sabina EP. Intl. J. PharmTech Res. 2016, 9(4):675–82.

This work is licensed under a Creative Commons Attribution 4.0 International License.









