Binding Behavior of Few Lanthanides and Heavy Metal ions with Proteins by Fast and Reliable Affinity Capillary Electrophoresis Method
Hassan A. Alhazm and Mohammed Al-Bratty
Department of Pharmaceutical Chemistry, College of Pharmacy, Jazan University, Jazan, Post code 45142, Saudi Arabia.
Corresponding Author E-mail: haalhazmi@jazanu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/330619
The interaction of two lanthanides Cerium (Ce3+) and Gadolinium (Gd3+) and two heavy metal ions Manganese (Mn2+) and Zinc (Zn2+) with various biological proteins Bovine Serum Albumin, Human Serum Albumin, β-lactoglobulin, Myoglobin and Ovalbumin were studied by using mobility shift affinity capillary electrophoresis. The used ACE method offers fast operation using shorter capillary, small injection volume, adequate short rinsing protocol and lower concentration of samples. The normalized difference of mobility ratio (ΔR/Rf) was used to investigate the possible interaction. All interactions were summarized as ΔR/Rf chart and compared. For heavy metal ions, the interactions of Mn2+ with all proteins were significant with negative ΔR/Rf values except with Myoglobin (MB) as its ΔR/Rf close to zero (0.002± 0.03) and one of its cnf(ΔR/Rf) values intersect the zero line indicates insignificant interaction. Very weak interactions between Zn2+ and all proteins were detected with small +ΔR/Rf values and confidence intervals intersected the zero line. For lanthanides, Cerium (Ce3+) showed weak interactions with BSA, HAS. Whereas, strong interactions were observed with MB and BLACT. Gd3+ showed almost insignificant interactions with most of the tested proteins except MB, it showed strong interaction with MB in a manner similar to Ce3+. Influence of the coordination number of the metal ions and multiple binding sites within the proteins on the signs of ΔR/Rf values were discussed in details. Furthermore, the change in protein peak shape was found to be as evident for a possible conformational change for the proteins and their affinity to the metal ions.
KEYWORDS:Affinity capillary electrophoresis; lanthanides; heavy metal ions; mobility shift; protein-metal ion interaction
Download this article as:| Copy the following to cite this article: Alhazmi H. A, Al-Bratty M.Binding Behavior of Few Lanthanides and Heavy Metal ions with Proteins by Fast and Reliable Affinity Capillary Electrophoresis Method. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Alhazmi H. A, Al-Bratty M.Binding Behavior of Few Lanthanides and Heavy Metal ions with Proteins by Fast and Reliable Affinity Capillary Electrophoresis Method. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40306 |
Introduction
Metalloproteins
Proteins that have a specific metal ion as cofactor are called as Metalloproteins. A large number of proteins are metalloproteins and all these require metal ion for their function [1, 2]. Popular protein-metal ion interactions are known to produce various crucial biological roles such as storage as ferritin for Fe3+, metallothionein proteins for Zn2+, Cu2+ and other heavy metals, transport as transferrin for Fe3+ and oxygen transport by binding to Fe2+ of heme metalloprotein, cofactor for enzymes e.g. selenium on glutathione peroxidase. Furthermore, Cu2+, Mn2+, Fe2+ and Ni2+ can be incorporated to form active sites of antioxidant defense enzymes such as superoxide dismutases and Mg can be incorporated for hexokinase [3-5]. The toxicity of some of the heavy metals such as Hg2+ and As3+ can be manifested when they bind irreversibly to a variety of selenocystine enzymes [6-8]. The investigation of diagnostic biomarkers for several diseases such as metalloproteins is still growing [1]. Furthermore, a substantial progress in producing organometallic complexes for several disorders such as cancer, inflammation, infection and neurological disorders have been achieved [9-11]. The influences of metal ion partner should be investigated prior to developing new prodrugs. Therefore, the characterization of interactions between proteins and metal ions are important.
HSAB Concept for Study Protein-Metal Ion Interaction
The hard and soft acids-bases concept (HSAB) helps us to understand the interaction behaviors between metal ions and biological proteins [12, 13]. In general, the metal ions interact with the protein binding sites of similar hardness or softness preferentially [12]. Figure 1 was successfully drawn to summarize most of hard, borderline and soft acid metal ions and base functional groups. Many of base functional groups within Figure 1 are involved in the structure of the amino acids and hence protein.
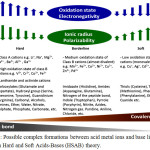 |
Figure 1: Possible complex formations between acid metal ions and base ligands based on Hard and Soft Acids-Bases (HSAB) theory. Click here to View figure |
Investigated Proteins
Bovine Serum Albumin (BSA), Human Serum Albumin (HSA), β-lactoglobulin (BLACT), Myoglobin (MB) and Ovalbumin (OVA) were selected for the study because of their important role in various functions of the body and they are known to interact with metal ions. In mammals, Serum albumins have been found to be associated in the control of the biologically active concentrations of Ca2+ and Mg2+ [14]. The reactivity and binding ability of BSA with a variety of monovalent and polyvalent metal ions such as Cu2+, Ni2+, Co2+, Pt2+, Cd2+, Pb2+ has been investigated [15-18] earlier. Similarly β-lactoglobulin was also reported to have affinity towards a number of metal ions such as Li+, Na+, Cu2+ and Zn2+ that also modulate its thermal aggregation. [19-21]. The interaction study of myoglobin with Co2+, Zn2+ and Cu2+ was carried out by using UV-Visible spectroscopy by Tang et al [22], whereas, ovalbumin was also reported to have significant interactions with various metals such as Cu2+, Ni2+ ions [23] and Cd2+ ions [24] that was investigated by Immobilized metal ion affinity chromatography (IMAC), equilibrium dialysis method respectively as well as by affinity capillary electrophoresis [12].
Investigated Metal Ions
The behaviors of two lanthanide ions Cerium (Ce2+) and Gadolinium (Gd2+) and two heavy metal ions Manganese (Mn2+) and Zinc (Zn2+) on some biological proteins are not studied yet by using mobility shift- affinity capillary electrophoresis (mobility shift-ACE). Therefore, they were selected for study owing to their importance in current and future drug therapy. The lanthanide series of elements are categorized as hard acids and they form ionic or electrostatic bonds with other elements largely. Various studies related to the interaction of Ce2+ with biological proteins have been carried out [25, 26]. The other lanthanide Gd2+ is toxic in biological systems as it competes with Ca2+ ions for all the processes that need Ca2+ for proper functioning, probably due to the fact that both ions have similar degree of hardness. It is extensively used to prepare contrast agents for Magnetic Resonance Imaging (MRI). Gd2+ interactions with various proteins and amino acids are studied nowadays and this idea is used to prepare protein based contrast agents recently [27-29].
On the other hand, the heavy metal ion Mn2+ is bound to metalloproteins, mainly glutamine synthetase present in astrocytes [30, 31] and acts as a cofactor for various enzymes inside the body. Similarly Zn2+ is known to interact with a variety of enzymes and amino acids like cysteine, histidine (-N), aspartate (-O), and glutamate (-O) residues [32] and some hormones like Prolactine [33]. It also plays role as a cofactor for various enzymes to act properly.
Mobility Shift-Affinity Capillary Electrophoresis
Affinity Capillary Electrophoresis (ACE) is a free solution Capillary Electrophoresis (CE) method [34]. In this method the change in electrophoretic migration time or peak (area or height) for one of the reactants (an analyte or a ligand) before and after binding is used to investigate the interaction [35, 36]. ACE has received wide popularity in recent years being used in biological and pharmaceutical research [37-39]. Recently, ACE was effectively utilized for the investigation of the interaction between ovalbumin isoforms as well as few other proteins and various metal ions [40, 41].
In mobility shift-ACE, the sample for injection contains an analyte and an EOF marker while the running buffer contains a ligand in varying concentration. Under the electric field, two peaks will be detected which are corresponding to the EOF marker and the analyte. The migration time of the analyte (e.g. protein) peak can be changed after binding to a ligand (e.g. metal ion). Therefore, this parameter is used for binding calculation. The use of an EOF marker is important to avoid calculation errors due to the possible change of the EOF during experiments due to some spontaneous and uncontrolled factors such as fluctuation on room temperature, possible metal ion and protein adsorptions on capillary wall.
The mobility shift-ACE was selected in this work due to many advantages. It is very sensitive method since a very weak interaction can be detected by the migration time change of 0.1 s [40, 41, 12]. Furthermore, the small change in the migration time is more obvious when the overall charge of a protein is changed by a metal ion and other surrounding anions. It allows to work with very small amount of an analyte, e.g. at the level of limit of detection (LOD) since the binding investigation is based only on the migration time. On the other hand, ligands with no UV absorption can be used. It has the ability for simultaneous investigation of interactions of a number of analytes with metal ions within one sample (mixture or even impure) due to its high separation power. Recently, this method has been used for the screening of interaction of an analyte (protein) with a ligand (metal ion) [35]. The screening was based on using of a high concentration of a ligand to achieve the saturation [38-39]. Recently, Alhazmi et al has successfully published a mobility shift-ACE platform chart for a wide screening interactions between five proteins and 28 metal ions [12]. However, behaviors of many element ions with the selected proteins are still not studied by mobility shift-ACE.
Materials and Methods
Chemicals and Reagents
Human serum albumin (HSA, 97%), Bovine serum albumin (BSA, 99%), β-Lactoglobulin (BLACT, 85% bovine milk), Myoglobin (MB, 90%) and Ovalbumin (OVA, 98%) were procured from Sigma-Aldrich (Steinhim, Germany). Metal ions Cerium (III) chloride (CeCl3, 99.99%), Gadolinium (III) chloride (GdCl3, 99.99%), Manganese (II) chloride (MnCl2, 99.99%) and Zinc chloride (ZnCl2, 99.99%) were also procured from Sigma-Aldrich (Steinhim, Germany). Acetanilide was purchased from Fluka (Steinhim, Germany), whereas, Disodium Ethylene diamine tetraacetic acid dihydrate (EDTA-Na2.2H2O) and sodium hydroxide (NaOH) were obtained from Riedel de Häen (Hannover, Germany). Concentrated hydrochloric acid (Conc. HCl) was obtained from Merck (Darmstadt, Germany) and double distilled water was prepared in our laboratory.
Apparatus and Instrumentation
A recently developed, very fast and reliable mobility-shift ACE method was used for investigation of metal ion interactions with the selected proteins. The electrophoretic separations were carried out on Agilent CE system (model G1600A; Agilent Technologies, Germany) that consists of an autosampler, a diode array detector and a capillary cooling system. Normal air plug was utilized to apply high pressure. Bare fused silica capillaries were purchased from Polymicro Technologies (Phoenix, USA) having I.D. 50 μm, total length 31 cm (short capillary) with an effective length of 22 cm. Rotilabos-syringe filters were purchased from Carl Roth (0.22 mm, CME, Karlsruhe, Germany). The pH of the buffer solutions used was adjusted by using Mettler Toledo pH-meter (FE20/EL20, Carl Roth, Germany). The collected data were interpreted by using the installed ChemStation software (Agilent). Finally, the binding calculations and their statistical analysis were done on Microsoft EXCEL™ (Microsoft Corporation, version 2013) using the mobility ratios of protein before and after addition of metal ions in running buffer.
Rinsing Protocol
The conditioning of new capillaries were performed at a pressure of 1 bar first by using 1 N sodium hydroxide for 20 min and then with double-distilled water for 10 min. At the start and end of each working day, capillaries were flushed again at 2.5 bar by using 0.1 N NaOH for 10 min followed by water for 5 min. At the beginning of each run, capillaries were rinsed at 2.5 bar for 2.5 min with a solution contains 0.1N sodium hydroxide water and 0.1 M EDTA, for 1 min and running buffer for 1.5 min. After each screening, the capillary was rinsed at 2.5 bar with 0.1 N NaOH solution for 10 minutes followed by water for 5 minutes to remove adsorbed traces from proteins.
Separation Conditions
Normal mode in which anode was present at inlet and cathode was present at outlet, was selected for the separations. Temperature maintained was 23 °C and voltage applied was 10 kV. Hydrodynamic injections of samples were carried out at 50 mbar for 4.5 s, except for MB, as it was injected for 1.5 s since the short injection time of 1.5 s improved the electrophoretic separation between the EOF marker and MB. Running buffer was injected after the injection of samples at 50 mbar for 2.5 s to push the samples. A total of twelve runs were performed for the screening of each metal-protein interaction. Six runs were conducted for protein alone and six for proteins along with metal ions.
Preparation of Solutions
Tris buffer was prepared with concentration of 20 mmol/L having pH 7.4. For the preparation, 2.42 g of tris was dissolved in 200 mL double distilled water. The pH 7.4 was adjusted with HCl and volume was made up to 1 L with double distilled water. Acetanilide was used as EOF marker as it remains neutral at pH 7.4 [41, 42]. The pKa of Acetanilide is 0.5 as the non-bonded electrons of nitrogen get delocalized through the resonance of the conjugative system. 37.5 mg of acetanilide was dissolved in 50 mL of tris buffer followed by sonication to get a concentration of 750 μg/mL. All the metal ion and protein solutions were prepared fresh in tris buffer every working day. The protein solutions were prepared in definite concentrations in tris buffer separately. Various concentrations of proteins were, BSA (20 μmol/L), HSA (20 μmol/L), BLACT (50 μmol/L), MB (60 μmol/L) and OVA (50 μmol/L) by dissolving an amount of 33 mg of BSA, 33.18 of HSA, 23 mg of BLACT, 25.8 mg of MB and 53.5 mg of OVA in a mixture of stock solution of acetanilide (5 mL) and making up the volume to 25 mL with tris buffer. Before carrying out the experiments, all protein solutions were diluted three times in order to reduce band broadening as well as protein adsorption. The solutions of metal ions were prepared in the same tris buffer of concentration 20 mmol/L and pH 7.4 to obtain the concentrations of 25, 100 and 250 μmol/L.
Results and Discussion
Binding Calculation
The mobility ratios Ri and Rf of the test protein were calculated with and without interaction with ligand metal ions respectively, using an EOF marker, by the equation R = teof /tprot, where teof is the migration time for EOF marker (Acetanilide) and tprot is the migration time for test protein. The normalized difference between the mobility ratios (Ri-Rf)/Rf or ΔR/Rf were used to represent the interaction results.
The confidence interval of ΔR/Rf (cnfΔR/Rf ) was calculated by equation:
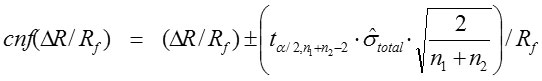
For a given degree of freedom, tα/2 is the t-value at probability 0.975 (α/2 = 0.025). The n1 and n2 are the two data numbers of the series to estimate Ri and Rf. The value n1+n2-2 is the degree of freedom.
Total standard deviation,
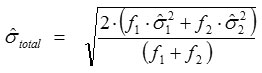
where ƒ1 and ƒ2 are the numbers of freedom (n-1) where as
![]()
and
![]()
are the standard deviations of the data of two series for Ri and Rf, respectively.
Interaction of Metal Ions with Proteins
The binding behavior of each group of metals (Table 1) on the selected proteins, BSA, HSA, BLACT, MB and OVA was studied under physiological pH 7.4. Two Lanthanide group metal ions (Ce3+ and Gd3+) and two heavy metal ions (Mn2+ and Zn2+) were examined. As we know that multiple sites are exhibited by most of proteins for different metal ions, therefore, each metal ion was used in high concentrations for fast interaction screening with protein in order to attain saturation [41, 42]. Metal ion concentrations of around 250 μmol/L was proved to be suitable for all the tested proteins except for BLACT and MB, because of their intensive change in peak shapes that were not able to get integrated when they are used in concentrations above 100 μmol/L for BLACT [41, 42] and above 25 μmol/L for MB. Six repeated runs were performed for each solution and the migration time data (see Fig. 2 as example) were used for the calculation of ΔR/Rf values and their confidence intervals to measure the interaction strength using equations 1, 2 and 3. All obtained ΔR/Rf values and their cnfs are summarized in Table 1. Indeed, it is difficult to compare between metal ions by using this Table. Hence, ΔR/Rf chart (Fig. 3) was successfully created to make comparisons easier.
 |
Figure 2: Typical electropherograms of EOF marker (acetanilide, 1st migrated peak) and proteins (2nd migrated peak) BSA, HSA, BLACT, MB and OVA without (solid line) and with (dotted line) Mn2+, Zn2+, Ce3+ and Gd3+ in the running buffer. |
Separation conditions: capillary I.D. 50 μm and total length 31 cm with effective length 22 cm, buffer tris (pH 7.4) 20 mmol/L, sample injection for 4.5 s at 50 mbar (for MB 1.5 s) followed by buffer (2.5 s), voltage 10 kV, UV 214 nm, capillary cartridge temperature at 23 °C. Rinsing at 2.5 bar using solution containing 0.1 N NaOH and 0.1 M EDTA for 2.5 min, water for 1 min followed by tris buffer for 1.5 min. Extra flushing post each screening at 2.5 bar with 0.1 N NaOH solution for 20 min followed by water for 10 min.
Table 1: ΔR/Rf values with their confidence intervals of all investigated protein-metal ion interactions
|
Metal ions |
Proteins |
|||||
|
BSA |
HSA |
BLACT |
MB |
OVA |
||
|
Heavy Metals |
Mn2+ |
-0.067 ± 0.008 |
-0.068 ± 0.006 |
-0.135 ± 0.019 |
0.002 ± 0.003 |
-0.098 ± 0.015 |
|
Zn2+ |
0.013 ± 0.003 |
0.005 ± 0.007 |
0.013 ± 0.003 |
0.009 ± 0.002 |
0.008 ± 0.003 |
|
|
Lanthanides |
Ce3+ |
-0.016 ± 0.031 |
-0.082 ± 0.081 |
-0.125 ± 0.007 |
-0.392 ± 0.027 |
-0.034 ± 0.066 |
|
Gd3+ |
-0.007 ± 0.021 |
-0.00002 ± 0.011 |
0.008 ± 0.002 |
-0.102 ± 0.021 |
-0.012 ± 0.037 |
|
The negative and positive values of ΔR/Rf are of great importance as far as the preliminary evaluation of protein-metal ions interactions is concerned. It gives insight into the coordination of metal ion bound with the residues of protein and surrounding anions. For example, the estimated initial charge of BSA in the running buffer, is -18 at pH 7.4 [38] in absence of metal ion ligand whereas, when a metal ion ligand is added in the buffer, the charge of BSA generally decreases to ≤ -18 (less negative) that leads to increased electrophoretic mobility resulting in a positive ΔR/Rf value as in case of BSA-Zn2+. On the other hand, some metal ions especially those which have high coordination numbers and are bound to BSA could further coordinate with some of the anions around and that will cause an increase in the charge of BSA ≥ -18 (more negative). In these cases, the electrophoretic mobility would be decreased resulting in a negative ΔR/Rf value as in case of BSA- Mn2+ (see Fig. 3).
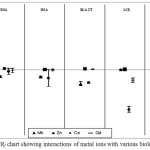 |
Figure 3: ΔR/Rf chart showing interactions of metal ions with various biological proteins |
Heavy Metal Ions
The interactions of heavy metal ions (Mn2+ and Zn2+) selected for study were found to be different for different proteins. For Mn2+ the interaction was in negative direction in most of the cases except Myoglobin (MB). The interaction of MB was not significant as their ΔR/Rf value < 0.01 and closed to zero and one of its cnf(ΔR/Rf) values intersect the zero line (0.002±0.003). With other proteins, Manganese ion was found to interact comparatively more and led to the decrease in the electrophoretic mobility and hence negative values were obtained. Strongest interaction was observed in case of BLACT (ΔR/Rf ± cnf = -0.135 ± 0.019) followed by OVA > HSA ≥ BSA. On the other hand the other investigated ion Zn2+ showed very weak or could be insignificant interactions with all the tested proteins as their confidence intervals intersected the zero line. Interestingly all the small ΔR/Rf values in case of Zinc ion was found to be positive for all the proteins. This indicates that, Zinc ions could decreased the overall charge of proteins and made it less negative, therefore, increased the electrophoretic mobility that resulted into positive ΔR/Rf values.
Interaction of metal ions with proteins had an interesting effect on peak shape and intensity and it changed with the interaction. The change in the protein peak shape is also due to the conformational change of the protein [12]. Manganese ion (Mn2+) interaction was significant and in negative direction in case of proteins BSA, HSA, BLACT and OVA and it was evident from the change in peak shape and intensity in their corresponding electropherograms (Fig. 2). Peaks corresponding to the complexes Mn-BSA and Mn-HSA were observed to be higher in intensity and broader in shape, whereas, in case of BLACT, peak with metal ion was broader but significantly lesser in intensity owing to the good interaction of Mn2+ with BLACT. Mn2+ did not show any significant interaction with MB and it was confirmed by the peaks obtained with and without metal ion as there was no any significant change in shape or intensity was observed. If we consider the electropherogram of Mn2+-OVA, splitting of peak was observed in case of protein with metal ions, which is because of the separation of two isoforms OVA-A and OVA-B. Zinc ions (Zn2+) displayed no significant interaction with all the proteins and the electropherograms displayed no significant change in peak shape or intensity in all the cases.
Lanthanides
The interactions of lanthanides Cerium (Ce3+) and Gadolinium (Gd3+) with various proteins were found to be interesting, especially with the Myoglobin (MB) protein. Ce3+ showed weak interaction with BSA, HSA, and OVA and the ΔR/Rf values were typically in the negative direction for all the proteins and their confidence intervals cross the zero lone . Whereas, strong interactions were observed between Ce3+ ions and MB showing ΔR/Rf values in negative direction (-0.392 ± 0.027) as well as with BLACT (ΔR/Rf = -0.125 ± 0.007). This may be attributed to the high coordination numbers of Ce3+ ion [1, 8] that allows it coordinate with other surrounding ions present resulting in the increase in negative charge of the protein and decrease in the electrophoretic mobility. The other lanthanide metal ion investigated was Gd3+ that showed almost insignificant interactions with most of the tested proteins except MB. Gd3+ also showed strong interaction with MB in a manner similar to Ce3+, and in this case the ΔR/Rf values were also in negative direction demonstrating that the negative charge of protein was increased by Gd3+ ions that led to the decrease in electrophoretic mobility. Hence various binding sites present on MB and further coordination with the surrounding anions should be taken into account when considering Ce3+ and Gd3+ based drugs. Interactions of Gd3+ with BSA, HSA, BLACT and OVA were very weak and the ΔR/Rf remained near the zero line with non-significant values. Again there is some possibility of interaction that was present there but it could not be detected by the instrument. Over all, the most significant interaction was observed between lanthanide metal ions Ce3+ and Gd3+ and MB, whereas, other tested proteins showed weak affinity towards these metal ions except BLACT that showed good interaction with Ce3+ also.
If we observe the change in peak shape and intensity in case of lanthanides with proteins, Cerium (Ce3+) displayed insignificant change in peaks corresponding to Ce3+-BSA, Ce3+-BLACT and Ce3+-OVA whereas for Ce3+-HSA and Ce3+-MB, the change was highly significant with broader peak in case of Ce3+-HSA and broader and higher intensity peak for Ce3+-MB with elevated baseline (the right side of peak was not touching the baseline). Gadolinium (Gd3+) showed significant interaction with MB only and it was evident from the peak obtained corresponding to Gd3+-MB that was much broader and elevated not touching the baseline. The significant change on MB peak shape could be due to the strong influence on the protein conformation. No significant change in peaks was obtained for other proteins as no significant interaction was observed between Gd3+ and other proteins (Fig. 2).
Conclusions
ACE is becoming more popular nowadays especially for the investigation of protein-metal ion interactions. In this work, an optimized and fast ACE method was developed successfully with lower sample concentration, smaller injection volume and a proper rinsing protocol. Several factors were further considered in order to improve the precision. These were sample pushing, shorter rinsing protocol, extra flushing after several consecutive runs and getting buffer solutions at inlet and outlet refreshed. In order to get good long term precision, 0.1 M EDTA was used in the rinsing protocol. The optimized conditions that were finalized was injection of sample at a pressure of 50 mbar for a period of 4.5 s that was pushed with the help of buffer at 50 mbar for a period of 2.5 s. Rinsing protocol was shortened with solutions of 0.1 N NaOH and 0.1 M EDTA at 2.5 bar for a period of 2.5 min followed by water and running buffer for 1 min and 1.5 min respectively. Some extra flushing was also employed after completing every 60 runs, at the same pressure by using 0.1 N NaOH solution for 10 min followed by water for 5 min. Buffer solutions were regularly refreshed to improve the precision of the results after every 30 consecutive runs. The ΔR/Rf values were found to be different for different metal ions. In some cases it was found to be significant and in positive or negative direction showing good interaction while in other cases the interaction was very weak and can be considered as non-significant. These could probably be owing to the capability of coordination of the metal ions and positions of their binding sites. For example, binding sites at the surface of the protein could facilitate coordination with the background anions leading to negative ΔR/Rf, while binding sites inside the protein could facilitate coordination with potential ligands (amino acid residues) leading to positive ΔR/Rf. The change in peak shapes of proteins after binding with metal ions have been successfully used to detect the conformational change of protein due to influence of metal ions.
Acknowledgement
The authors are thankful to Prof. Dr. Hermann Wätzig, Institute of Medicinal and Pharmaceutical Chemistry, Braunschweig, Germany, for his valuable guidance throughout this research work.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Swart, C. Anal Bioanal Chem. 2013, 405, 5697–5723.
CrossRef - Mounicou, S.; Szpunar, J.; Lobinski, R. Chem Soc Rev. 2009, 38, 1119–1138.
CrossRef - Lodish, H.; Berk, A.; Matsudaira, P.; Kaiser, C.A.;, Krieger, M. Molecular Cell Biology, 2003, 5th edn. W. H. Freeman, New York.
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. J Biol Inorg Chem. 2008, 13, 1205–1218.
CrossRef - Sun, H.; Chai, Z. Annu Rep Prog Chem Sect A. 2010, 106, 20–38.
CrossRef - Carvalho CML, Chew E, Hashemy SI, Lu J, Holmgren A, J Biol Chem 2008.,283: 11913-11923.
CrossRef - Romero-Canelon, I.; Sadler, P.J. Inorg Chem. 2013, 52, 12276−12291.
CrossRef - Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Dou, Q.P. Curr Pharm Des. 2010, 16, 1813–1825.
CrossRef - Chen, D.; Milacic, V.; Frezza, M.; Dou, Q.P. Curr Pharm Des. 2009, 15, 777–791.
CrossRef - Hannon, M.J. Pure Appl Chem. 2007, 79, 2243–2261.
CrossRef - Desoize, B. Anticancer Res. 2004, 24, 1529-1544.
- Alhazmi, H.A.;, Nachbar, M.; Albishri, H.M.; El-Hady, D.A.; Redweika, S.; El Deeb, S.; Wätzig, H. J Pharm Biomed Anal. 2015, 107, 311–317.
CrossRef - Wiberg, N.; Wiberg, H. Inorganic Chemistry, 2001, English Version, first edn. Academic Press, California.
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Mol Immunol. 2012, 52, 174–182.
CrossRef - Liu, H.; Shi, X.; Xu, M.; Li, Z.; Huang, L.; Bai, D.; Zeng, Z. Eur J Med Chem. 2011, 46, 1638–1647.
CrossRef - Sathyadevi, P.; Krishnamoorthy, P.; Jayanthi, E.; Butorac, R.R.; Cowley, A.H.; Dharmaraj, N. Inorganica Chimica Acta. 2012, 384, 83–96.
CrossRef - Gharagozlou, M.; Boghaei, D.M. Spectrochim Acta A Mol Biomol Spectrosc. 2008, 71, 1617–1622.
CrossRef - Klotz, I.M.; Urquhart, J.M.; Fiess, H.A. J Am Chem Soc. 1952, 74, 5537–5538.
CrossRef - Navarra, G.; Leone, M.; Militello, V. Biophys Chem. 2008, 131, 52-61.
CrossRef - Navarra, G.; Tinti, A.; Foggia, M.D.; Leone, M.; Militello, V.; Torreggiani, A. J Inorg Biochem. 2014, 137, 64-73
CrossRef - Beierlein, F.R.; Clark, T.; Braunschweig, B.; Engelhardt, K.; Glas, L.; Peukert, W. J Phys Chem B. 2015, 119, 5505–5517
CrossRef - Tang, Q.; Zheng, X.F.; Wang, J.Y.; Liu, Y.Y.; Yuan, Y.L. Guang Pu Xue Yu Guang Pu Fen Xi. 2009, 29, 1958-1961.
- Sharma, S.; Agarwal, G.P. Anal Biochem. 2001, 288, 126-140.
CrossRef - Verma, S.R.; Arora, J.P.S.; Shankar, J.S.; Dutt, D.; Pal, C. Water Air Soil Poll. 1989, 43, 53-59.
CrossRef - Ali, M.; Kumar, A.; Kumar, M.; Pandey, B.N. Biochimie. 2016, 123, 117-129.
CrossRef - Kumar, A.; Ali, M.; Ningthoujam, R.S.; Gaikwad, P.; Kumar, M.; Nath, B.B.; Pandeya, B.N. Radiation; J Hazardous Mat. 2016, 307, 281–293.
CrossRef - Shenghui, X.; Jingjuan, Q.; Fan, P.; Mathew, C.; Jenny, Y. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2013, 5, 163–79.
CrossRef - Li, S.; Jiang, J.; Zou, J.; Qiao, J.; Xue, S.; Wei, L.; Long, R.; Wang, L.; Castiblanco, A.; White, N.; Ngo, J.; Mao, H.; Liu, Z.R.; Yang, J.J. J Inorg Biochem. 2011, 107, 111–118.
CrossRef - Caravan, P. Acc Chem Res. 2009, 42, 851-62.
CrossRef - Takeda, A. Brain Res Rev. 2003, 41, 79–87.
CrossRef - Holeysovska, H. Collect Czech Chem Commun. 1961, 26:, 3074-3080.
CrossRef - Alhazmi, H.A.; El Deeb, E.; Nachbar, M.; Redweik, S.; Albishri, H.M.; El-Hady, D.A.; Watzig, H. J Sep Sci. 2015, 38, 3629-3637.
CrossRef - Pace, N.J. Eranthie Weerapana E Biomolecules. 2014, 419-434.
CrossRef - Neubert, R.H.H.; Rüttinger, H.H. Affinity Capillary Electrophoresis in Pharmaceutics and Biopharmaceutics. 2003, Marcel Dekker Inc., New York.
CrossRef - Albishri, H.M.; El Deeb, S.; Al-Garabli, N.; Al-Astal, R.; Alhazmi, H.A.; Nachbar, M.; El-Hady, D.A.; Wätzig, H. Bioanalysis. 2004, 6, 3369-3392.
CrossRef - Wätzig, H.; Degenhardt, M.; Kunkel, A. Electrophoresis. 1998, 19, 2695-2752.
CrossRef - Chu, Y.; Avila, L.Z.; Gao, J.; Whitesides, G. Acc Chem Res. 1995, 28, 461-468.
CrossRef - Redweik, S.; Xu, Y,.; Wätzig, H. Electrophoresis, 2012, 33, 3316–3322.
CrossRef - Redweik, S.; Cianciulli, C.; Hara, M.; Xu, Y.; Wätzig, H. Electrophoresis. 2013, 34, 1812–1819.
CrossRef - Mironov, G.G.; Logie, J.; Okhonin, V.; Renaud, J.B.; Mayer, P.M.; Berezovski, M.V. J Am Soc Mass Spectrom. 2012, 23, 1232-1240.
CrossRef - Heegaard, N.H.H.; Nilsson, S.; Guzman, N.A. J Chromatography B. 1998, 715, 29–54.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









