Synthesis and Characterization of Manganese, Copper and Zinc Complexes Derived from Schiff-Base Ligand
Birendra Kumar1, Bishwanath Kumar2, Sunny Kumar1, Deepak Kumar3 and Shivadhar Sharma3
1Department of Chemistry, Jagjiwan College, Gaya, India.
2Department of Chemistry, S D College, Paraiya, India.
3Department of Chemistry, Magadh University, Bodh-Gaya, India.
Corresponding Author E-mail: birendra5556@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330563
A Schiff base has been prepared by the condensation of 4- amino -3,5-dimercapto,1,2,4-triazole with 2,4-diaminobenzaldehyde. The ligand has been used for complexation with Mn(II), Cu(II) and Zn(II) metal ions. The complexes have been characterized by their elemental analysis, molar conductivity, magnetic susceptibility, IR spectra and electronic spectra. The very low value of molar conductivity of complexes shows their non-electrolytic nature. The comparison of IR spectra of complexes with that of free ligand reveals that the ligand is coordinated through azomethine nitrogen and deprotonated thiolsulphur forming five membered heterochelates. The magnetic moment values and electronic spectral bands clearly indicate that octahedral geometry around metal ions in complexes.
KEYWORDS:Heterochelates; Non- electrolytic; Schiff Base
Download this article as:| Copy the following to cite this article: Kumar B, Kumar B, Kumar S, Kumar D, Sharma S. Synthesis and Characterization of Manganese, Copper and Zinc Complexes Derived from Schiff-Base Ligand. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Kumar B, Kumar B, Kumar S, Kumar D, Sharma S. Synthesis and Characterization of Manganese, Copper and Zinc Complexes Derived from Schiff-Base Ligand. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=36486 |
Introduction
The schiff base transition metal complexes have invited the attention of Inorganic Chemists because of vast application in pharmaceutical and industrial fields.(1-5)Due to their simple synthesis and versatility Schiff complexes continue to remain important and a popular area of research as such complexes persistently play a verysignificant role in understanding the various aspect of coordination chemistry of transition metals. (6-10) The schiffbase of triazole derivatives have been found pharmaceutically active.( 11-13) The pharmaceutical activity of these schiffbase have been found to get enhanced on complexation with metal ions.14 The literature reveals that schiffbase complexes of 4- (2/,4/-diaminobenzaldimino)-3,5-dimercapto-1,2,4-triazole has not been taken care of. Therefore, in continuation of our previous work,15 in the present paper we report the synthesis and characterization of Mn(II), Cu(II) and Zn(II) complexes with Schiff base, 4- (2/,4/-diamino -benzaldimino)-3,5-dimercapto-1,2,4-triazole.
Material and Method
All the reagents used in the present work were of Anal. R grade and were used as received. Thiocarbo –hydrazide, carbon disulphide, α- picoline, pyridine, ethanol, 2,4- diaminobenzaldehyde were procured from Merck (India) and metal salts of Mn(II), Cu(II) and Zn(II) procured from Lova Chemic (India ). For the preparation of the ligand at first the pyridinium salt of 4- amino-3,5-dimercapto-1,2,4-triazole was prepared by refluxing the mixture of thiocarbohydrazide and carbon disulphide in pyridine on oil bath for one hour. Thereafter, the pyridinium salt was treated with hydrochloric acid to get 4- amino-3,5-dimercapto-1,2,4-triazole. This amine was refluxed with 2.4-diaminobenzaldehyde in ethanol in the presence 2 drops concn sulphuric acid for one hour to get yellow crystals of schiff base, i.e., 4- (2/,4/-diaminobenzaldimino)-3,5-dimercapto-1,2,4-triazole. This ligand was used for complexation with metal chloride in ethanolic medium by usual method of reflux by adding 2 ml of α- picoline. On cooling the reaction mixture the solid complex was separated out which was filtered and washed with alcohol. It was dried in desiccator over anhydrous calcium chloride. The C, H and N elemental analysis were carried out using Perkin Elmer 2400 II elemental analyzer. IR spectra of ligand as well as complexes were recorded on Perkin- Elmer FTIR spectrophotomet- er (spectrum II) using KBr pellet. The magnetic moment of complexes were determined by Gouy balance method at room temperature. Determination of molar the conductivity of complexes with 10-3 solution in DSMO was done usingElico direct reading Conductivity meter. The electronic spectra of complexes were recorded on Perkin- Elmer Lamda 950 spectrophotometer. Results are given in Table I
Table: 1
|
Compound |
%M Calc (Found) |
%C Calc (Found) |
%H Calc (Found) |
%N Calc (Found) |
%S Calc (Found) |
µeff (B.M.) |
Conductivity moh cm-1 mole-1 |
|
LH |
— (–) |
42.857 (42.902) |
3.968 (4.051) |
27.778 (27.805) |
25.397 (25.401) |
||
|
[MnL2(α-pico)2] |
7.375 (7.405) |
48.326 (48.352) |
4.564 (4.581) |
22.552 (22.538) |
17.183 (17.201) |
5.96 |
16.20 |
|
[CuL2(α-pico)2] |
8.432 (8.425) |
47.775 (47.485) |
4.512 (4.506) |
22.295 (22.307) |
16.986 (16.978) |
2012 |
15.48 |
|
[ZnL2(α-pico)2] |
8.654 (8.634) |
47.659 (47.672) |
4.501 (4.488) |
22.241 (22.245) |
16.945 (17.013) |
— |
14.00 |
LH = 4- (2/,4/-diaminobenzaldimino)-3,5-dimercapto-1,2,4-triazole α-pico=α- Picoline, Calc = Calculated
Result and Discussion
The molar conductivity values of complexes are indicative of their non electrolytic nature.16-17 On the basis of elemental analysis and molar conductivity values the complexes are formulated as [ML2(α-pico)2], where L is the schiff base ligand. IR spectra of complexes are very cumbersome and hence only important bands have been assigned and explained. The IR spectra of free ligand display two bands at 3013 and 2859 cm-1 which are assigned to νasym NH2 and νsym NH2 stretching vibration.18 These two bands do not undergo any appreciable change in their frequencies in the spectra of complexes. It shows no coordination of NH2 group to the metal ions. The band at 30 10cm-1 is assigned to νCH aromatic group while the band at 2950 cm-1 is fairly assigned to νCH of azomethine group.19-21 The strong band at 2560 cm-1 is assigned to νSH stretching vibration of the ligand. In the spectra of complexes this band appears at lower frequency with highly decreased intensity. It shows that out of two SH group one has undergone deprotonation and coordination occur though deprotonated mercapto sulphur.22 The other major change is found in the absorption frequency of azomethine group of the free ligand which absorbs at 1630 cm-1 in the spectrum of free ligand and shifts to lower frequencies by 30-40 cm-1 in the spectra of the complexes. It is indicative of coordination through azomethine nitrogen of ligand. The coordination through deprotonated mercaptosulphur and azomethine nitrogen is further confirmed by appearance of new bands at 510 and 370 cm-1 due to νM-N and νM-S stretching vibration respectively in complexes. The new band appears at 755 cm-1 in the spectra of complexes which shows the presence of coordination of α-picolinein complexes.24-28
The magnetic moment of Mn (II) complex is determined to be 5.96 B.M. which corresponds to 5 unpaired electrons. It shows that Mn (II) complex is paramagnetic and it is high spin complex. 29-32Mn(II) complex displays three bands in its electronic spectra which may be assigned to spin forbidden transition as below :- 19500 cm-1 (ν1)=6A1g→4T1g(4G), 23480 Cm-1 (ν2)= 6A1g→4T2g(4G) and 19500 cm-1(ν3)= 6A1g→4Eg(4D). As the transitions are spin forbidden and also Laporte forbidden the intensity is very very poor. Using Tanabe – Sugano diagram the various crystal field parameters have been calculated with values
![]()
B=844.15 cm,-1Dq= 1063.63 cm.-1 So B0=0.137, as according to Orgel the energy of 4G lies above 6A1g by 17B +5C. So 17B +5C = ν3. From this the value of C is calculated to be 3029.89 cm.-1 Hence,
![]()
which is very close to theoretical value
![]()
(3.8) for Mn (II) complexes. The values of various crystal field parameters are in good agreement with the reported value for octahedral complexes of Mn (II).28,33-34
The magnetic moment of Cu (II) complex is found to be 2.12 B.M. at room temperature which shows it is magnetically dilute octahedral complex.35-38The electronic spectra of Cu (II) complex exhibit a broad band at 20350 cm-1 due to 2Eg→2T2g spin allowed transition. The broadness of band clearly indicates the further splitting of both 2Egand 2T2g due to departurate of symmetry from Oh to D4h symmetry. However, the value is in good agreement with the reported value of distorted Oh complexes of Cu (II).5,39-43
Zn (II) complex is diamagnetic which is in accordance with its d10 configuration. Hence it doesn’t display any band in electronic spectra. However, on the basis of elemental analysis and molar conductivity Ohstructure is assigned to Zn (II) complex
The tentative structure of complexes may be given below:-
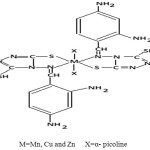 |
Scheme 1 |
Acknowledgement
One of the authors Mr. Bishwanath Kumar is thankful to the Principal, M.G. College, Gaya for carrying out research work in its P.G. Department laboratory.
References
- More, P.G.; Bhalvankar, R.B.; Patter, S.C. J. Indian Chem. Soc.,2001, 78, 474
- Jayabalakrishanan,J.; Natrajan, K. Synth.React.Inorg.Metal-Org.Chem.,2001, 31, 983
- Ispir, E.; Kortoglu, M.; Purtas, F.; Serin, S. Transition Met- Chem.,2005, 30, 1042
- Sari, N.;Nartop, D.; Logoglu, E. Asian J.Chem.,2009, 21, 42331
- Kumar, D.; Sharma, J.; Chadda, S.; Symal, S. J. Indian Chem. Soc.,2014, 91,185-193
- Correa, W.H.; Scott, J.L. Molecules,2009, 513
- Sukasi, C.; Pakawatchi, C.; Thipyapoong, K. Polyhedron,2008, 27, 759
CrossRef - Taylor, M.K.; Trotter, K.D.; Reglinski, J. Berlouis. L.E.A.; Kennedy, A.R.; Spickett, C.M.; Sowden, R.J.J. Inorg. Chim.Acta.,2008, 361, 9
- Karmakar, T.K.; Ghosh, B.K.; Osman,A.;Fun, H.K.; Mallah, T.M.; Aromi,G.; Chandra, S.K. Inorg. Chem.,2005, 44, 2391
CrossRef - Triphathy, S.K.; Panda, A.; Dash, P.K.; Behra, N.K.; Panda, A.K. J. Indian Chem. Soc.,2016, 93, 275
- Piotr, P.; Adam, H.; Krystian, P.; Bogmil, B. and Franz, B. Curr. Org. Chem, 2009,13,124
CrossRef - Ghosh , S.; Malik, S.; Jain, B.; Gupta, M. J. Indian Chem. Soc.,2012, 89, 471.
- Bala, M ; Mishra, L.K. J. Indian Chem. Soc.,2014, 90,143
- Patil, S.; Bodiger,B.M.; Kudari, S.N.; Kulkarni, V.RTransition Metal Chem.,1983, 45, 879
- Kumar, B.; Kumar, R.; Kumar, B. Orient. J. Chem.,2015, 31(3), 1827
CrossRef - Mahapatra,B.B.; Patel, N. J. Indian Chem. Soc.,2009, 86, 518
- Ramaswami, I.; Ramaswami, S. J. Indian Chem. Soc.,2014, 61, 1877
- Bandopadhyay, N.; Lu, L.; Zhu, M.;Bhattacharya, R.; Nashkar, J.P. J. Indian Chem. Soc.,2015, 92, 15
- Singh, M.K.; Lasker, A.D.R.; Paul, B. J. Indian Chem. Soc. 2008,85, 485.
- Chandra, S.; Jain, D.; Sarkar, A. J. Indian Chem. Soc. 2009,86, 220
- Saha, N. Indian J Chem. 1981,20A, 680
- Sharma Y.R., Elementary Organic Spectroscopy, S. Chand & Co. Pvt. Ltd. New Delhi,Revised Edition, (2013)
- Dubey,R.K.; Mishra, S.K.; Mariya, A.; Mishra, A.K. J. Indian Chem. Soc., 2013,90,29
- Fraerzer ; Glowacky .J. Am. Chem. Soc. 1948,70, 2575
- Yamada, H. Appl. Spectrosc.Rev.1981,17, 227
CrossRef - Gill, N.S.; Kingdom,H.J. Aust. J Chem.1966,19,2197 27.Liver, A.B.P.; Ramaswami, B.S. . Indian Canad.J Chem. 1973,51, 1582
- Joseph,A.; Joseph,B.; Narayana,B. J. Indian Chem. Soc. 2008,85, 479
- Mukharjee, A.K.; Roy, P. J. Indian Chem. Soc.,1955, 32, 633
- Saccni, L.; Cimr, R. Annal.chem. Italy,1952, 42, 723
- Bhatnagar, S.S.; Nevgi, M.B.; Sharma, R.L. Phil.Mag.,1936 , 22, 409
CrossRef - Guha, B.C. Proc. Roy.Soc.London,1951, A-206, 353
CrossRef - Kumari, P.; Prakash, S.; Prakash, D. J. Indian Chem. Soc.,2012, 89, 19
- Siddappa, K.; Reddy, P.C.; Kotem, M.; Reddy, T.;Tanbe, M.; Mitra, M.Asian J. Chem.,2011, 23, 4511
- Patel, R.N.; Sukla, K.K.; Singh, A.Chaudhari, N.M.; Chouhan, U.K.;Dwedi, S. Inorg. Chem. Acta,2009, 362, 4891
CrossRef - Patel, R.N.; Singh, N.; Gundla, V.L.N. Polyhedron,2007, 26, 257
CrossRef - Borthakur, R.; Kumar, A.; Lal, R.A. J. Indian Chem. Soc.,2014, 91, 407
- Rawat, S.P.; Choudhary, M. J. Indian Chem. Soc.,2014, 91, 491
- Pamja, M.; Pragathi, J.; Kumari, C.J. J.Chem. Phem. Res.,2011, 3, 602
- Mishra, A.P.; Gupta, P.; Jain, P.K. J. Indian Chem. Soc.,2013, 90,867
- Suryawanshi, N.J.; Pethe, G.B.; Bhadange, S.G.; Aswar, A.S. J. Indian Chem. Soc.,2013, 90, 1327
- Bartaria, D.; Singh, M.; Krishna, V. J. Indian Chem. Soc.,2013, 90, 1341
- Aswar, A.S.; Bansod, A.B.; Aswale, S.R.;Mandlik, P.R. Indian J. Chem.,2004, sec A-43, 1892

This work is licensed under a Creative Commons Attribution 4.0 International License.









