Taft Equation - A Convenient Tool to Decide the Position of Attack in the Reactions of Aliphatic Amines and Tl (III)
R. Sanjeev1, V. Jagannadham2, Adam A Skelton3, Pandiri Sreedhar4, V. E. M. Mamatha Bethapudi1 and R. Veda Vrath3
1Department of Chemistry, Geethanjali College of Engineering and Technology, Cheeryal, Medchal District, 501301, Telangana, India.
2Department of Chemistry, Osmania University, Hyderabad-500007, India.
3Department of Pharmacy, School of Health Science, University of Kwazulu Natal, Durban South Africa.
4Department of Chemistry, L N Gupta Evening College, Hyderabad-500002, India.
Corresponding Author E-mail: rachuru1sanjeev1@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/330517
Amines; Oxidation; One Electron Oxidant and Two Electron Oxidant
Download this article as:| Copy the following to cite this article: Sanjeev R, Jagannadham V, Skelton A. A, Sreedhar P, Bethapudi V. E. M. M, Vrath R. V. Taft Equation - A Convenient Tool to Decide the Position of Attack in the Reactions of Aliphatic Amines and Tl (III). Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Sanjeev R, Jagannadham V, Skelton A. A, Sreedhar P, Bethapudi V. E. M. M, Vrath R. V. Taft Equation - A Convenient Tool to Decide the Position of Attack in the Reactions of Aliphatic Amines and Tl (III). Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=36529 |
Introduction
The Hammett equation, which is based on the linear free energy relation, does not apply to the reactions of aliphatic compounds and ortho substituted benzene derivatives. This is because of interference of substituent by the reaction center. Also Hammett’s σ values concern groups attached to an aromatic system engaged in resonance.
For aliphatic compounds, the Taft equation in simple form is described as log k = log ko + σ* ρ* where k = rate constant for a particular member of a reaction series, ko = rate constant for the parent compound, r* = polar reaction constant and σ* is the polar substituent constant which is the measure of the electron attracting ability of the substituent. It is a purely inductive effect and transmits itself through the aliphatic chain. It is obtained by dividing the total effect of the substituent into steric and polar contributions effect. Taft, further, assumed that the reaction center interacts with substituents, which is the sum of polar effects and steric effects.
If log (k/ko) vs σ* is a straight line (with slope equal to r*), it indicates that the series of the substituted compounds follow the same reaction mechanism. A non-linear plot suggests that the compounds within a series do not adopt the same mechanism. This equation can also be utilized for finding the site of attack in amines. This is discussed in Tl(III) oxidations in acetic medium and Cu(III) oxidations in basic medium.
Discussion
A literature survey revealed the work done on the kinetics of oxidation of aliphatic amines is scarce. The oxidation of amines by oxidants like permanganate2, lead (IV) acetate3, cobaltic perchloride4, N-bromosuccinimide5, chorine dioxide6 and Tl(III)7 have been carried out. Tl(III) in acetic acid medium has been used as an oxidizing agent in the oxidation of many organic compounds. In most cases, Tl(III) is converted to Tl(I) in a single step. However, oxidations are known where Tl(III) is converted to Tl(I) in two one electron steps and, therefore, it was thought worthwhile to find the site of attack of the oxidation of amines by Tl(III). The first order dependence of the rate, both on Tl(III) and ammines, and formation of products, NH3 and RCHO, can be explained by one of the following three schemes.
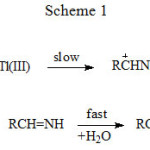 |
Scheme 1 |
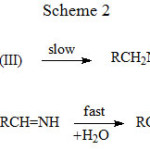 |
Scheme 2 Click here to View scheme |
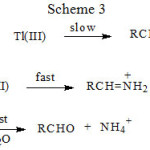 |
Scheme 3 |
The three schemes lead to the same products, but differ in the initial attack of Tl(III) on the aliphatic amine. In scheme 1 Tl(III) attacks RCH2NH2 at α-CH, in scheme 2 at NH and in scheme 3 on the lone pair of electrons on nitrogen. Where exactly the attack would take place can be decided by the application of Taft equation? The amines that were oxidized in acid medium were methyl amine, ethyl amine, n-propyl amine, n-butyl amine, iso-butyl amine and n-hexyl amine. If the oxidant attacked at α-CH, the corresponding substituents to the reaction site are shown in Table 1 and each substituent has an σ* value corresponding to it. Applying the Taft equation for this series of reactions, a plot is constructed, log k vs σ*. A straight line with a negative slope was observed, indicating general applicability of the Taft equation. The value of r*, as found from the graph was -5.08 with a good correlation coefficient of 0.9902, indicating the reaction is highly sensitive to the presence of the substituent and the reaction proceeds via an ionic mechanism. Thus, it can be concluded that the amines follow the Taft equation and the site of attack is α-CH (Table 1, Figure 1).
Table 1: Effect of substituents on k in Tl(III) – amine reaction assuming a-CH bond breaking
|
Sl.No. |
Amine |
R |
Substituents |
Σσ* |
k x 106 mol-1min-1 |
6 + log k |
|
1 |
methyl |
H |
H & H |
0.98 |
1.80 |
0.26 |
|
2 |
ethyl |
CH3 |
CH3 & H |
0.49 |
900 |
2.95 |
|
3 |
n-propyl |
CH3CH2 |
CH3CH2 & H |
0.39 |
1600 |
3.20 |
|
4 |
n-butyl |
CH3CH2CH2 |
CH3CH2CH2 & H |
0.37 |
5100 |
3.71 |
|
5 |
i-butyl |
(CH3)2CH |
(CH3)2CH & H |
0.30 |
6640 |
3.82 |
|
6 |
n-hexyl |
n-C5H11 |
n-C5H11 & H |
0.24 |
6800 |
3.83 |
[H2SO4] = 1.00 M, [HOAc] = 50% (v/v) m = 1.00 M maintained with KCl, temp. = 318 K
 |
Figure 1: Taft plot for the reation of amines with TI(III) assuming α-CH bond breaking Click here to View figure |
The study of the substituent effect also rules out scheme 2 involving N-H bond rupture. If this were to be the case, then the substituents for the methyl, ethyl, n-propyl, n-butyl, iso-butyl and n-hexyl amines would be themselves methyl, ethyl, n-propyl, n-butyl, iso-butyl and n-hexyl respectively (Table 2). Plot of log k vs σ* should be linear. But it was not linear (Figure 2). When methyl amine is taken as the standard instead of ethyl amine such linearity was not observed supporting scheme 1.
Table 2: Effect of substituents on k in Tl(III) – amine reaction assuming N-H bond breaking or attack of oxidant on lone pair of electrons of nitrogen. Conditions are same as in Table 1.
|
Sl.No. |
Amine |
R |
Substituents |
σ* |
k x 106 mol-1min-1 |
6 + log k |
|
1 |
methyl |
H |
CH3 |
0.00 |
1.80 |
0.26 |
|
2 |
ethyl |
CH3 |
CH3CH2 |
-0.10 |
900 |
2.95 |
|
3 |
n-propyl |
CH3CH2 |
CH3CH2CH2 |
-0.12 |
1600 |
3.20 |
|
4 |
n-butyl |
CH3CH2CH2 |
CH3CH2CH2CH2 |
-0.25 |
5100 |
3.71 |
|
5 |
i-butyl |
(CH3)2CH |
(CH3)2CHCH2 |
-0.13 |
6640 |
3.82 |
|
6 |
n-hexyl |
n-C5H11 |
n-C6H13 |
-0.28 |
6800 |
3.83 |
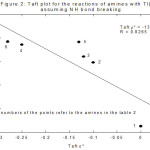 |
Figure 2: Taft plot for the reation of amines with TI(III) assuming NH bond breaking |
Scheme 3 involves abstraction of an electron from the lone pair of nitrogen, resulting in the formation of the radical cation RCH2NH2·+. Further no polymerization occurred, when acrylonitrile was added. If we assume that if the abstraction of lone pair of electrons on nitrogen occurred then ion with two positive charges on nitrogen would be present. Such a situation is not known. Even if the Taft equation is applied appropriately to scheme 3, again linearity is not observed and the plot would be similar to Figure 2. Hence the scheme 3 is also ruled out.
An important point with regard to the application of the Taft equation in Scheme 2 and Scheme 3 is that, the substituent linked to the reaction center (nitrogen atom) is the same in both cases. Hence the application of Taft equation cannot discern scheme 2 and scheme 3. Only the discussion put forth in the foregoing paragraph can distinguish scheme 3 from scheme 2.
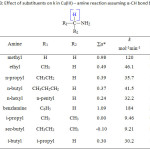 |
Table 3: Effect of substituents on k in Cu(III) – amine reaction assuming α-CH bond breaking |
Analogous to the above-mentioned discussion, the site of attack in oxidation of amines by
Cu(III) can also be decided using Taft equation. Table 3, Figure 3 (linearity is observed) and Table 4, Figure 4 (non-linear) and Table 5 (non-linear), Figure 5 are self-explanatory.
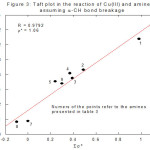 |
Figure 3: Taft plot in the reation of Cu(III) and amines assuming a-CH bond breaking Click here to View figure |
 |
Table 4: Effect of substituents on k in Cu(III) – amine reaction assuming NH bond breaking |
 |
Figure 4: Taft plot in the reation of Cu(III) and amines assuming N-H bond breaking Click here to View figure |
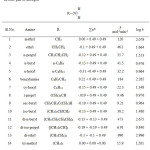 |
Table 5: Effect of substituents on k in Cu(III) – amine reaction assuming attack of the oxidant on lone pair of nitrogen Click here to View table |
 |
Figure 5: Taft plot in the reation of Cu(III) and amines assuming nitrogen lone pair attack Click here to View figure |
It will not be out of place to mention about the application of Taft equation to direct ester oxidation by Tl(III)8 where polar and steric effects are operative.
References
- Sanjeev, R..; Jagannadham, V.; Veda Vrath, R.. Chem Ed NZ, 2012,November 14
- Rawlay, S. S. and Schechter, H. J .Org. Chem., 1967, 32, 3129.
CrossRef - Resanvleatt, D. H.; Davis, G. T.,; Hull, L. A.and Forenberg, G. D. J. Org. Chem, 1968, 33, 31649.
- Shaik, R. A. and Waters, W. A. J .Chem. Soc.(B), 1970, 988.
- Sonia Dunstan and Handest, H.B. J. Chem. Soc.(B), 1957, 4905.
- Rosenblatt, D. H.; Hayes, A. J. (Jr.).; Harison, B. L.,; Streaty, R. A. and Moore, K. A. J. Org. Chem, 1962, 28, 2790.
CrossRef - Venkat subramanyam and Srinivas V.S. (Sr) Indian J. Chem., 1979, 18(3), 259
- Veda Vrath R.,; Sethuram, B and Navaneeth Rao, T., Indian J. Chem., 1979, 17A, 410.

This work is licensed under a Creative Commons Attribution 4.0 International License.









