Recovery of Palladium from Concentrated Nitrate Solutions with N,N′‐Dimethyl‐N,N′‐Dioctyltetradecylmalonamide as new Extractant
Emad A. Mowafy1,2 and D. Mohamed1
1Faculty of science, Hail University, Hail, P.O. BOX 2240, Saudi Arabia.
2Hot Labs. Centre, Atomic Energy Authority, P. O. Box 13759, Cairo, Egypt.
Corresponding Author E-mail: mowafy69@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330530
The new unsymmetrical malonamide namely; N,N′‐dimethyl‐N,N′‐ dioctyltetradecyl-malonamide (DMDOTDMA) was prepared and evaluated as agent for the extraction and separation of Pd(II) from nitrate media was studied. N,N′‐dimethyl‐N,N′‐dioctylmalonamide (DMDOMA) was synthesized and used for comparison purpose. The effect of various parameters had been studied in details to explain the extraction properties of pd(II) by using DMDOTDMA in mixed diluents (75 vol.% n-dodecane-25 vol.% n-octanol) from nitrate media. The extraction equilibrium of Pd(II) with the investigated maloamides was reached within 10 minutes. Pd(II) showed remarkable extractability by DMDOTDMA and the maximum extraction percentage of Pd(II) is nearly reached at ~ 5 M HNO3. Under similar extraction conditions Zn(II),Ni(II), Mn(II) Cu(II) and Pb(II) were found poorly extracted. The dominate extracted species of Pd(II) is Pd(NO3)2(MA)2.The separation of Pd(II) from other studied metal ions was found to be effective in the range of nitric acid solution used. The stripping of Pd(II) was successfully carried out by single contact with 0.1M thiourea solution.
KEYWORDS:Novel Extractant; Palladium; Base Metal Ions; Extraction/Separation; Nitric Acid
Download this article as:| Copy the following to cite this article: Mowafy E. A, Mohamed D. Recovery of Palladium from Concentrated Nitrate Solutions with N,N′‐Dimethyl‐N,N′‐Dioctyltetradecylmalonamide as new Extractant Orient J Chem 2017;35(5). |
| Copy the following to cite this URL: Mowafy E. A, Mohamed D. Recovery of Palladium from Concentrated Nitrate Solutions with N,N′‐Dimethyl‐N,N′‐Dioctyltetradecylmalonamide as new Extractant Orient J Chem 2017;35(5). Available from: http://www.orientjchem.org/?p=39297 |
Introducation
Palladium is one of the most important of the platinum group metals (PGMs) with high corrosion resistance and catalytic properties. It has different important applications in industrial fields such as automobile catalytic converters1-4. In fact, palladium is a very rare metal in the earth’s crust, the world wide reserves being localized in very few countries1,5. Since, the recycling of secondary resources such as electronic scrap and catalytic converters is very important. Therefore, the recycling of new secondary sources is mandatory for future supply of palladium5,6. In fact, liquid-liquid extraction is an important process in the recycling of the platinum- group metals (PGMs), and thus there are valuable efforts for the development of novel effective extractant molecules2,7,8. Several reagents have been used for the palladium extraction and most of them were developed during the last three decades and still in used today. Several classical common and/or commercial extractants like; Cyphos®IL-1019, dialkyl sulphoxides10, 4-acylpyrazolone11, 2-hydroxy-4-sec-octanpyl diphenyl-ketoxime12, Acorga®Clx50,8-hydroxyquinoline, tributylphosphate (TBP), Aliquat 336, Cyanex 923, Cyanex 471X13,14 have been used for the extraction of pd(II) from hydrochloric acid solutions. In general, these extractants have several problems like poor solubility in aliphatic diluents, slow kinetics of extraction, chemical stability, and pH sensitivity, etc15-17. In view of these limitations, it becomes necessary to develop of new classes of ligands. Many researchers have tried to improve and develop novel extractants, which have high extraction efficiency as well as selectivity for platinum group metals (PGMs). During the last twenty years a several of amide bidentate ligands have been often proposed for the solvent extraction process, mainly due to their faster kinetics, higher efficiency, selectivity, and complete incinerability18-22.
Very recently, a new class of ligands namely; malonamides, have come to play an important role in this subject. Although malonamides have been extensively investigated for the extraction of lanthanide and actinide ions in nuclear processing industry21-23, only a limited amount of work reported involving the use of these ligands for the extraction of Pd(II) from hydrochloric acid solutions 3,8. No systematic studies have been reported on the extraction of Pd(II) from concentrated nitrate media. Therefore, the development of new solvent extraction systems for the extraction and separation of Pd(II) from nitrate solutions is an important subject. The extraction properties of Pd(II) with malonamides from nitrate solutions have not yet been understood.
Recently, a few malomamides derivatives have been planned for solvent extraction of PGMs from hydrochloric acid solution3,24-27. Costa et al.,24 have used N,N’-dimethyl-N,N’-diccyclotetradecyl maloamide (DMDCHTDMA) to effective extraction of platinum chloride solutions. Platinum, palladium and rhodium extraction from hydrochloric acid media has been studied with N,N’-dimethyl-N,N’-diphenyltetradecylmalonamide(DMDPHTDMA)26. Poirot et al.5, investigated the extraction behavior of N,N’–dimethyl,N,N’-dioctylhexylethoxymalonamide (DMDOHEMA)/ n-heptane system towards palladium from nitrate media. They observed the formation of third phases due to the poor solubility of palladium complexes with DMDOHEMA/n-heptane system in different conditions.
To our knowledge, there is no available work involving the use of DMDOTDMA in nitric acid solution or hydrochloric acid solution for the extraction of Pd(II). No systematic investigation has been reported on the extraction of Pd(II) with DMDOTDMA from concentrated nitrate media. In a previous work1,2,23,28,29, we reported on the synthesis and utilizations of different diamides, diglycolamides and cyanamides for the extraction and separation of selected PGMs and base metal ions from various acid solutions.
In the present study, selective extraction of Pd(II) with DMDOTDMA in mixed diluents (75 vol.% n-dodecane-25 vol.% n-octanol) from nitric acid medium in the presence of metal ions, such as Co(II), Zn(II),Ni(II), Mn(II) Cu(II) and Pb(II) was carried out using binary and multicomponent mixtures. The effective separation of Pd(II) from other studied metal ions nitric acid solution was highlighted during this work. DMDOMA was studied for comparison purpose.
Experimental
DMDOMA and DMDOTDMA were synthesized by using the method described below. The investigated metal ions aqueous solutions were prepared by dissolving the desired metal nitrate in nitric acid solution. Different Pd(II) salt (palladium chloride) and other aqueous medium (hydrochloric acid) were also used. The stock solutions of the investigated metal ions(Pd(II) Co(II), Zn(II),Ni(II), Mn(II) Cu(II) and Pb(II)) were diluted to obtain the desired concentrations for working solutions. In this study, all reagents of analytical grade purity and used without additional purification.
Synthesis of DMDOMA and DMDOTDMA
N,N′‐dimethyl‐N,N′‐dioctylmalonamide(DMDOMA) and N,N′‐dimethyl‐N,N′‐ dioctyltetradecylmalonamide (DMDOTDMA) were synthesized and purified following the procedure that we described previously21,29.
DMDOMA synthesized by adding a stoichiometric quantity of malonyl dichloride with N-metyloctylamine in presence of methylene chloride and triethylamine as a basic medium. The intermediate compound, malonyl dichloride, was prepared through the reaction of thionyl chloride and malonic acid, with suffering immediately a subsequent reaction with N-methyloctylamine at about 5 oC. The reaction was refluxed for about 5 hours. After purification and evaporation of the diluents, the obtained crude product was distilled under reduced pressure to get pure product. The purity of the prepared DMDOMA was found to be ~99%.
DMDOTDMA has been synthesized by taking DMDOMA as a starting material. DMDOTDMA was obtained by alkylation of the central carbon atom of DMDOMA dissolved in toluene by the reaction with sodium hydride, and finally with bromotetradecane.
The reaction mixture was refluxed for about 30 hours. After completion of the reaction, the crude product was separated and the diluent was evaporated. The yield of the crude product was found to be 92%. The two synthesized extractants were characterized by using different analytical tools like IR, elemental analysis, and H-NMR. The purity of DMDOTDMA was found to be more than 98%.
General Extraction Procedure
In order to prevent the co-extraction of HNO3, which may have occurred during the extraction of metal ions, the solvents (organic phases) were preequilibrated with the same nitric acid concentrations as the aqueous phase studied. Extraction process were carried out by shaking equal volumes of aqueous and organic phases in a thermostated water bath adjusted to the desired temperature (25 ±1oC) for about 30 minutes. After phase separation by centrifugation, known aliquots of the aqueous phase was sampled for analysis. The distribution ratio (DM) was determined as the ratio between the concentration of metal ion in the organic and aqueous phases, Eq. (1):
DM = [M]org/ [M]aq (1)
Percentage extraction of element (%E) was determined by using the following equation:
%E = DM/DM +1 x 100 (2)
where M is the metal ion (Pd(II) Co(II), Zn(II), Ni(II), Mn(II) Cu(II) or Pb(II)). All the extraction experiments were conducted in triplicate. Normally 1.5 × 10−3 M of the desired metal nitrate at various nitric acid concentrations was used as feed solution. The concentration of the element in the organic phase was determined by subtracting the aqueous concentration from the initial aqueous concentration of the investigated metal ions. The concentrations of Pd(II) Co(II), Zn(II),Ni(II), Mn(II) Cu(II) and Pb(II) in the aqueous phases were determined before and after extraction process by using ICP-AES 8100, Shimadzu and atomic absorption spectrometry (AAS, Perkin– Elmer-400). The relative error values associated with all measurements were found to be about ± 2.5%. The extracted species of Pd(II) in the organic phase were studied by using FT-IR (Thermo Scientific Nicolet-6700), with the spectra recorded in the range 400–4000 cm–1.
Back Extraction Studies
For this study, the organic phase of DMDOMA or DMDOTDMA was loaded with 1.5 × 10−3 M Pd(II) in 5 M HNO3. Equal volumes of the loaded organic phase and aqueous phase containing the stripant agent were equilibrated in separator funnel using a mechanical shaker for 30 minutes.
Re-utilization of DMDOTDMA
To gain insight on the stability or regeneration characteristics and feasibility of reusability of this extractant, an organic phase containing DMDOTDMA in 75 vol.% n-dodecane-25 vol.% n-octanol was subjected to five successive extraction-stripping contacts with fresh solutions of Pd(II) in 5 M HNO3 and with 0.1M thiourea in 0.5M nitric acid, respectively.
Results and Discussion
Certain preliminary experiments were performed in order to explain the extraction behavior of palladium(II) by DMDOMA and DMDOTDMA in the absence of other the other investigated metal ions. The optimum extraction conditions of palladium(II) were established by various the experimental parameters, such as HNO3 concentration, DMDOTDMA concentration, and the extraction equilibration time.
The formation of solid third phases were obtained during the extraction of palladium by using DMDOHEMA/ n-heptane/ nitric acid system5.
In general, to avoid the third phase formation, a long-chain alcohol a polar reagent can be added into the organic solution. In this study, mixture of 75 vol.% n-dodecane-25 vol.% n-octanol was chosen as the diluent for the investigated malonamides extractants. For the both DMDOMA and DMDOTDMA, no third phase formation was obtained after extraction process; good and fast phase separation was also observed for all the extraction experiments system that was conducted.
Extraction Equilibrium Studies
The variation of percentage extraction (%E) of palladium(II) with DMDOMA or DMDOTDMA in 75 vol.% n-dodecane-25 vol.% n-octanol as a function of shaking time was investigated, Fig. 1. It was found that the extraction of palladium from aqueous solution was obtained in short period time ~ 10 minutes.
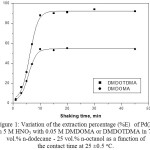 |
Figure 1: Variation of the extraction percentage (%E) of Pd(II) in 5 M HNO3 with 0.05 M DMDOMA or DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol as a function of the contact time at 25 ±0.5oC. Click here to View figure |
Influence of Acid Concentration on the Extraction of Palladium
Figure 2 shows the variation of the percentage extraction of Pd(II) with DMDOMA or DMDOTDMA as a function of HNO3 concentration. It can be seen that the extraction of Pd(II) with DMDOMA is dependent on the HNO3 concentration until about 5 M, after which a slight decrease was observed. For DMDOTDMA, the extraction of Pd(II) increases with increase in the initial concentration of HNO3 up to about 5 M. This followed by decrease of extraction until the range of HNO3 concentration studied, 7M. This may be attributed to the protonation of the extractant at higher HNO3 concentrations. The poor extraction of palladium in the very low concentration region of HNO3 solution with these malonamides permits an easy back-extraction of palladium(II) with lower HNO3 concentration solution.
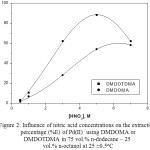 |
Figure 2: Influence of nitric acid concentrations on the extraction percentage (%E) of Pd(II) using DMDOMA or DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol at .25 ±0.5 oC |
Figure 3 showed the ability of DMDOMA or DMDOTDMA to extract Pd(II) from HCl solution. It can be seen that the extraction percentage of palladium(II) with DMDOMA is slightly affected by increasing HCl concentration from 0.5 to ~3 M, after which a sharp increase was observed. The extraction of Pd(II) with DMDOTDMA is decrease with the hydrochloric acid concentration until ~2 M, after which a sharp increase was obtained, as shown in Fig.3.
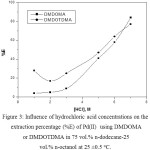 |
Figure 3: Influence of hydrochloric acid concentrations on the extraction percentage (%E) of Pd(II) using DMDOMA or DMDOTDMA in 75 vol.% n-dodecane-25 vol.% n-octanol at 25 ±0.5oC. Click here to View figure |
By comparison of the results obtained from Figs 2 and 3, it can be concluded that the extraction of palladium increases when alkylation the central carbon atom. The alkyl substituent in DMDOTDMA was found to cause a noticeable increase in the extraction ability for Pd(II) compared with the non-central alkylated analogue (DMDOMA). This effect was anticipated in our present work. So far, no available work was reported on the influence of the introduction of alkyl group on the central carbon atom for the extraction of palladium(II) from nitric acid solutions. In general, the extractability of the malonamides can be attributed to both the electroinductive influence as well as the steric hindrance around the active binding site. Therefore, the alkylation of central carbon atom in malonamide could have play an important role for palladium extraction from nitric acid or hydrochloric acid solutions.
In this concern, Mowafy and Aly29 investigated the extraction of iron(III) from HCl solution with other malonamides. They found that the alkylation of the central carbon atom suppressed the extraction of iron from HCl medium. However, further studies are required in this important area using various structures of malonamides to clarify the relationship between structures and the extraction of Pd(II).
Effect of Nitrate Ion Concentration on Palladium(II) Extraction
In order to explain the role of nitrate ion in the extraction reaction of Pd(II) with DMDOMA or DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol, the influence of nitrate ion on the distribution ratio of palladium(II) , DPd, was determined at fixed ionic strength. The concentration of nitrate ion was adjusted by using NaNO3. Fig. 4 shows that the relation between the distribution ratio of palladium(II), Dpd, and nitrate ion concentration is a straight line relationship with slope equal to ~2 for DMDOMA or DMDOTDMA. Therefore, two-nitrate ion participates in complex formation.
![Figure 4: Dependence of palladium(II) extraction on nitrate ion concentration with 0.05 M DMDOMA or DMDOTDMA in 75 vol.% n-dodecane - 25 vol.% n-octanol at 25 ±0.5 oC, [HNO3] = 1M.](http://www.orientjchem.org/wp-content/uploads/2017/10/Vol33No5_Rec_Ema_fig4-150x150.jpg) |
Figure 4: Dependence of palladium(II) extraction on nitrate ion concentration with 0.05 M DMDOMA or DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol at 25 ±0.5 oC, [HNO3] = 1M. Click here to View figure |
Composition of Pd(II) Extracted Species
Attempts were done to know about the nature of extracted complex species by studying the distribution ratios of Pd(II) as a function of DMDOMA or DMDOTDMA (MA) concentration. The extracted species composition can be ascertained from the relation between log DPd and log[MA] at 5 M HNO3, Fig. 5.
The graph of log DPd against log[MA] in 75 vol.% n-dodecane – 25 vol.% n-octanol was found to be liner, having a slope of 1.92 and 1.85 for DMDOMA and DMDOTDMA, respectively. Hence, this result suggests that the composition (metal:extractant) of the main extracted species of pd(II) from 5 M HNO3 by DMDOMA and DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol are 1:2. The obtained results are in agreement with those previously reported for the extraction of Pd(II) using other diamides1,5.
Therefore, we can suggest that the dominate extracted species of Pd(II) by DMDOMA or DMDOTDMA (MA) in vol.% n-dodecane – 25 vol.% n-octanol at 5 M nitric acid are Pd(MA)2(NO3)2. This can be represented by the following equilibrium (Eq.3):
Pd2+(aq) + 2 NO–3 (aq) + 2 MA (o) = Pd(NO3)2(MA)2 (o) (3)
where aq = aqueous phase, o = organic phase, and MA denotes the malonamides (DMDOMA or DMDOTDMA).
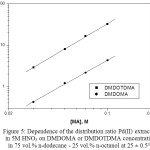 |
Figure 5: Dependence of the distribution ratio Pd(II) extraction in 5M HNO3 on DMDOMA or DMDOTDMA concentrations in 75 vol.% n-dodecane – 25 vol.% n-octanol at 25 ± 0.5°C. Click here to View figure |
Spectroscopic Studies
To provide insight into the bonding of the extracted species of Pd(II) by DMDOMA and DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol, FT-IR spectra of the organic phase were studied before and after metal ions extraction. Comparison of the IR spectra of the organic phase loaded with palladium(II) and pure DMDOMA or DMDOTDMA have been carried out.
The absorption band at 1644 and 1645 cm–1 can be attributed to the carbonyl-stretching mode in free DMDOMA and DMDOTDMA, respectively. A new vibration bands at 1625 and 1622 cm-1 were observed in the IR spectra of the extracted species. These two bands can be assigned to DMDOMA and DMDOTDMA, respectively, coordinated in the inner sphere, suggesting that DMDOMA and DMDOTDMA are coordinated to palladium through two carbonyl oxygen atoms. A shift of 19 and 23 cm–1 in the carbonyl group frequency for DMDOMA and DMDOTDMA, respectively, suggests stronger binding of these compounds with palladium, which is in agreement with the extraction results.
The peaks at 1378 and 1370 cm–1 in the IR spectra of the extracted species suggest the presence of free nitrate ions, which may exist in the extracted molecules20,28. These results are consistent with those reported previously by us and others for different diamides1,5,20,28.
Back-Extraction
To strip Pd(II) from organic phase 0.05 M (DMDOMA or DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol, water and different concentrations of diluted nitric acid were tried. The back-extraction of palladium(II) was found to be more difficult in all systems. On the other hand, water or dilute nitric acid are not sufficient as stripping agent for Pd(II) from organic phase. A solutions of (0.01. 0.05 and 0.1 M) thiourea in 0.5 M nitric acid was also used as the stripping agent for Pd(II) from the organic phase. It was found that 0.01 M thiourea was the most sufficient for palladium(II) stripping in one single contact. The recovered palladium(II) was found to be about 91% and 98% for DMDOMA and DMDOTDMA, respectively.
Extraction of Palladium(II) from Binary Solutions
The separation of palladium(II) from different elements in binary mixtures was attempted using DMDOMA or DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol. Table 1 shows high extractability of pd(II) with almost negligible extraction of Zn(II), Ni(II), Cu(II), Mn(II) and Pb(II).Thus, very high separation factor can be obtained for pd(II) over these elements. The results showed that pd(II) was always extracted at nearly constant value in the presence of the above elements for all the binary element mixtures systems, as shown in Table 1.
On the other hand, the extractability of Pd(II) with DMDOMA or DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol was not affected by the amount of elements in the binary mixtures used.
Table 1: Binary separation of Palladium(II) from Zn(II),Ni(II), Mn(II) Cu(II) and Pb(II) by 0.05 M DMDOMA or DMDOTDMA in vol.% n-dodecane – 25 vol.% n-octanol at 25±0.5oC; [HNO3] = 5 M.
|
Composition |
Molar ratio |
% Recovery of Pd(II) or M(II) |
|||
| DMDOMA |
DMDOTDMA |
||||
|
Pd(II) |
M(II) |
Pd(II) |
M(II) |
||
| Palladium(II) : Zinc(II) | 1 : 11 : 51 : 10 |
55.01 54.82 54.32 |
NE <0.001 <0.01 |
88.83 88.81 88.29 |
NE NE <0.001 |
| Palladium(II) : Nickel(II) | 1 : 11 : 51 : 10 |
55.04 54.42 54.23 |
<0.001 <0.01 <0.01 |
88.74 88.70 88.21 |
NE NE <0.001 |
| Palladium(II) : Manganese(II) | 1 : 11 : 51 : 10 |
54.95 54.71 54.15 |
<0.001 <0.01 0.1 |
88.55 88.31 88.18 |
<0.001 <0.001 <0.001 |
| Palladium(II) : Copper(II) | 1 : 11 : 51 : 10 |
54.72 54.20 54.16 |
0.02 0.02 0.04 |
88.72 88.20 88.06 |
<0.001 <0.001 ~0.01 |
| Palladium(II) : Lead(II) | 1 : 11 : 51 : 10 |
54.92 54.48 54.13 |
0.01 0.03 0.04 |
88.92 88.68 88.31 |
<0.002 0.003 0.02 |
Where 1:1 = 1.5×0.001M Pd(II): 1.5×0.001M M(II); 1: 5 = 1.5×0.001 M Pd(II): 7.5 x0.001M M(II); 1: 10= 1.5×0.001 M Pd(II):15x 0.001M M(II), M(II) = Zn(II),Ni(II), Mn(II) Cu(II) and Pb(II), NE= Not extraction.
Selectivity Patterns
Selectivity tests were carried out with DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol, for separation of palladium(II) from multicomponent mixtures.
Table 2 shows the high extraction of pd(II) from multicomponent mixtures of elements, viz. Zn(II),Ni(II), Mn(II) Cu(II) and Pb(II) with DMDOTDMA.
On the other hand, the results indicated the superior extraction of palladium(II) over other associated multi-component elements when extracted with DMDOTDMA. The high selectivity of DMDOTDMA is considered to have very interesting results in view of a practical application, considering that no significant contamination of the organic phases occurs.
Table 2: Selective extraction of 0.001 M Pd(II) from multicomponent mixtures of 0.005 M of each of Zn(II), Ni(II), Mn(II),Cu(II), Cu(II) and Pb(II) by 0.05 M DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol from different HNO3 concentrations.
| [HNO3],M |
% Extraction |
|||||
|
Pd |
Zn |
Ni |
Mn |
Cu |
Pb |
|
|
0.5 |
4.31 |
NE |
NE |
NE |
ND |
NE |
|
1 |
13.90 |
NE |
NE |
NE |
NE |
ND |
|
3 |
69.43 |
<0.001 |
NE |
ND |
ND |
<0.001 |
|
5 |
92.21 |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
~0.001 |
|
7 |
81.60 |
<0.001 |
<0.001 |
<0.001 |
~0.005 |
~0.003 |
where; NE= Not extraction and Nd= Not detection
Reusing of DMDOTDMA
To evaluate the stability and feasibility of reusing DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol for the selective recovery of palladium(II) from nitric acid for the adopted experimental conditions, experiments were also carried out.
Figure 6 shows five successive extraction–stripping cycles done with 0.05M DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol followed by striping with 0.1M thiourea in 0.5M nitric acid, using fresh 5 M HNO3 aqueous solutions of 0.001 M Pd(II) extraction in each stage.
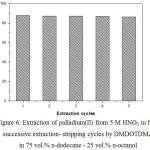 |
Figure 6: Extraction of palladium(II) from 5 M HNO3 in five successive extraction- stripping cycles by DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol. |
It was found that the extraction percentage of Pd(II) for five cyclic of extraction and stripping remained almost constant at the original value of DMDOTDMA. The obtained results clearly demonstrate the stability of DMDOTDMA and that re-utilization is feasible. On the other hand, no degradation of the DMDOTDMA in the used experimental conditions seems to occur. In this context, the results obtained in this study and that reported by others3,5,18,25,26 reinforces the possibility of successive reutilization of DMDOTDMA, which can probably be extended to other unsymmetrical malonamides derivatives, in view of their industrial application.
Conclusions
The present study offer an insight about a new solvent extraction application for unsymmetrical malonamide derivative namely; N,N’–dimethyl-N,N’– diotcyltetradecylmalonamide (DMDOTDMA) as a promising extractant for palladium(II). DMDOMA was investigated for comparison purpose. The extractability of Pd(II) with DMDOTDMA is higher than that of DMDOMA. The extraction equilibrium was reached within 10 minutes. The maximum extraction percentage of Pd(II) with DMDOTDMA is nearly reached at about 5 M HNO3.The composition (metal ion: extractant) of the dominate extracted species of Pd(II) from 5 M HNO3 by DMDOMA or DMDOTDMA in 75 vol.% n-dodecane-25 vol.% n-octanol are 1:2. Palladium(II) is found to be nearly quantitatively recovered from the organic phase by simple contact with 0.1 M thiourea in 0.5 M nitric acid. The results indicated that, the extractability of Pd(II) with DMDOTDMA in 75 vol.% n-dodecane – 25 vol.% n-octanol was not affected by the presence of elements in binary or multicomponent mixtures. Thus, this extractant (DMDOTDMA) could be a potential candidate for the selective extraction and separation of Pd(II) from other metal ions present in the waste nitrate liquors and also as alternative to commercial organophosphorous extractants.
Acknowledgements
The financial support kindly provided by Research Deanship, University of Hail, Saudi Arabia (project#0150115) is gratefully acknowledged.
References
- Mowafy, E.A.; Mohamed, D.; Alshammari, A. Sep. Sci. Tech. 2015, 50, 2352-2359.
- Mowafy, E. A.; Aly, H. F. J. Hazard. Mater. 2007,149, 465-470.
CrossRef - Paiva, A. P.; Carvalho, G. I.; Costa, M. I.; da Costa, A. M. R.; Nogueira, C. Sep. Sci. Tech. 2014, 49, 951-966.
CrossRef - Yamaguma, R.; Yamashita, A.; Kawakita, H.; Miyajima, T.; Takemura, C.; Ohto, K.; Iwachido, N. Sep. Sci.Tech. 2012, 47, 1303-1312.
CrossRef - Poirot, R., Bourgeois, D., Meyer, D. Solvent Extr. Ion Exch. 2014, 32, 529-542.
CrossRef - Cox, M. Solvent Extraction in Hydrometallurgy, in Principles and Applications of Solvent Extraction, 2nd Ed., Rydberg, J.; Musikas, C.; Choppin, G.R., Eds., Marcel Dekker Inc., New York, 2004, pp. 455-505.
- Ghasem, A. M. H.; Jadian, S. K. Orient. J. Chem. 2015, 31(1), 113-120.
CrossRef - Malik, P.; Paiva, A.P. Solvent Extr. Ion Exch. 2010, 28, 49-72.
CrossRef - Cieszynska, A.; Wisniewski, M. Sep. Purif. Technol.2010, 73, 202-207.
CrossRef - Preston, J.S.; Preez, A. C. du. Solvent Extr. Ion Exch. 2002, 20, 359-374.
CrossRef - Zhang, A. ; Wanyan, G. ; Kumagai, M. J. Solution Chem. 2004, 33, 1017-1028.
CrossRef - Shen,Y.F.; Xue, W. Y. Sep. Purif. Technol. 2007, 56, 278-283.
CrossRef - Bernardis, F. L; Grant, R. A.; Sherrington, D. C. React. Funct. Polym. 2005, 65, 205-217.
CrossRef - Gupta, B.; Singh, I. Hydrometallurgy. 2013, 134, 11–18.
CrossRef - Al-Bazi, S. J.; Freiser, H. Solvent Extr. Ion Exch.1987, 5: 265-275.
CrossRef - Dakshinamoorthy, A.; Dhami, P.S.; Naik, P.W.; Dudwadkar, N. L.; Munshi, S. K.; Dey, P.K.; Venugopal, V. Desalination. 2008, 232, 26-36.
CrossRef - Amara-Rekkab, A., Didi, M.A., Orient.J. Chem. 2015, 31(1), 205-214.
CrossRef - Nirita, H.; Tanaka, M.; Morisaku, K. In proceeding of 17th international solvent extraction conference “ISEC2005”, Beijing, China, September, 2005, 19-23, pp. 227-232.
- Mowafy, E. A.; Mohamed, D. Sep. Puri. Technol, 2014, 128, 18-24.
CrossRef - Mohamed, D.; Alshammri, A.; Mowafy, E.A. Radiochemistry. 2015, 57, 514-520.
CrossRef - Mowafy, E. A.; Aly, H. F. Solvent Extr. Ion Exch. 2002, 20, 177-194.
CrossRef - Mahajan, G. R.; Prabhu, D. R.; Manchanda, V. K.; Badheka, L. P. Waste Management.1998, 18, 125-133.
CrossRef - Mowafy, E. A. Radiochim Acta. 2007, 95, 539-545.
CrossRef - Costa, M. C.; Ana Assuncao, A.; Rosa da Costa, A. M.; Nogueria, C.; Paiva, A. P. Solvent Extr. Ion Exch. 2013, 31, 12-23.
CrossRef - Malik, P.; Paiva, A. p. Solvent Extr. Ion Exch.2009, 27, 36-49.
CrossRef - Malik, P.; Paiva, A.P. Solvent Extr.Ion Exch. 2008, 26, 25-40.
CrossRef - Nirita, H.; Morisaku, K.; Tanaka, M. Chem Commun. 2008, 45, 5921-5923.
CrossRef - Mowafy, E.A.; Aly, H. F. Solvent Extr. Ion Exch. 2007, 25, 205-224.
CrossRef - Mowafy, E. A. Solvent Extr. Ion Exch. 2007, 25, 1-18.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









