Activated Carbon Supported Cobalt as Efficiency Adsorbent: Application Chemical Agricultural Polluant 2,4-D Herbicide Removal from Aqueous Solution
Imen Boughaita1, Chafia Bouchelta1, Mohamed Salah Medjram1 and Pierre Magri2
1Laboratory LGCES, Faculty of Technology, 20 Août 1955 University, El-Hadaeik Road, P.O. Box 26, Skikda 21000, Algeria.
2Laboratory LCP-A2MC, University of Lorraine, 1 boulevard Arago, Metz, France.
Corresponding Author E–mail: boughaita.imen@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330510
In this work, an activated carbon derived from date pits and stems (DPSAC) was prepared by physical activation under N2 and used as a support of cobalt transition metals, by wetness impregnation at different Co content and different calcination temperatures. The optimal operator conditions were 20 wt% cobalt impregnation ratio and 300 °C calcination temperature. The grafted material was characterized by methods: thermogravimetric analysis (TGA), scanning electron microscopy (SEM), Fourier transform of infrared spectroscopy (FTIR). The activated carbon (DPSAC) and grafted activated carbon (Co/DPSAC) were realized on adsorption of 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solution , it was found that the cobalt-grafted dates pits and stems activated carbon promoted the 2,4-D adsorption kinetics, The Langmuir and Freundlich isotherms models were applied for the adsorption results. The rate of adsorption of 2,4-D onto (DPSAC) and (Co/DPSAC) were estimated and described using a pseudo-second order model.
KEYWORDS:Adsorption; Cobalt Grafted Activated Carbon; Impregnation; ( 2,4-D); Co/DPSAC; Dates Pits and Stems
Download this article as:| Copy the following to cite this article: Boughaita I, Bouchelta C, Medjram M. S, Magri P. Activated Carbon Supported Cobalt as Efficiency Adsorbent: Application Chemical Agricultural Polluant 2,4-D Herbicide Removal from Aqueous Solution. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Boughaita I, Bouchelta C, Medjram M. S, Magri P. Activated Carbon Supported Cobalt as Efficiency Adsorbent: Application Chemical Agricultural Polluant 2,4-D Herbicide Removal from Aqueous Solution. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=39402 |
Introduction
The pesticides in agricultural are applied to increase the profitability and quality of products; however, soils and water sources can become contaminated with these pesticides [1]. Chlorinated phenoxyacetic acid herbicides have been used as common weed killers. These elements are similar to the hormones of the natural plant and have become significant source of environmental pollutants [2]. 2,4-Dichlorophenoxyacetic is the more acidic herbicide used with high toxicity. 2,4-D is considered as a potential carcinogen and mutagen and persistent environmental pollutants by the International Agency for Research on Cancer, and is one of the common known endocrine disrupting chemicals [3]. 2,4-D may have other possible negative effects on human and animal health and fresh water ecosystems [4]. The recommendation of the World Health Organization (WHO) for the maximum admissible concentration of 2,4-D is 70 g/L in drinking water[3] . Some methods have been published for the removal of organic pollutants and pesticides from water [5]. Those methods include: oxidation, reverse osmosis, ion exchange, Electro-dialysis, electrolysis and adsorption. Many adsorbents have been developed for organic pollutants removal [1]. Activated carbon (AC) is one of these adsorbent, they can be made from nearly any organic material rich in carbon and preferably with low content in inorganic matter. In the last years, many agricultural materials are used and still receiving renewed attention such as date stones, esparto grass, peach stones, pomegranate seeds, rice straw, pistachio nut shells, apricot stones, grain sorghum, cherry stone, corncob and some other agricultural by products [6,7,8,9]. Generally, in laboratory scale, the preparation of activated carbon cans either chemical or physical activation. The chemical activation process is in general performed by one- or two-step activation methods. In the first one (one-step), a raw material is impregnated with a dehydrating agent (KOH, K2CO3, NaOH, Na2CO3, AlCl3, ZnCl2, MgCl2, H3PO4 and H2SO4) and the impregnated material is heat-treated under inert atmosphere [10]. The physical activation process is generally conducted in two stages: decomposing the hydrogenated matter by pyrolysis at high temperature and activation under gas flow in order to open the porosity. For mild oxidation of the carbonaceous material In this process, steam nitrogen, carbon dioxide or both of them are [11]. One of the most well-established and effective techniques for the removal of 2,4-D and pesticides from water is adsorption on activated carbons [12,13,14] . A wide range of adsorbents have been used for the removal of the 2,4-D : fungal Penicillium chrysogenum biomass [15] ; a prepared adsorbent from groundnut shell [14]; activated carbon made of langsat empty fruit bunch [16] ; oil palm frond activated carbon [17] ; banana stalk activated carbon [18] ; Modified granular activated carbon[19]; modified jute[20] etc. Great efforts have been made on development of multi-functionalized activated carbons, such as impregnation of functionalized metal ions/metal oxides on carbon surface. This kind of activated carbons can remove organic matters and cationic/anionic contaminants in the same time [21] .However, the transition metals as (Mn; Fe; Co; Ni; Cu; Zn; etc.) have yet been mentioned in the literature as potential of catalyst.
So in this study, we have first used dates pits and stems, an Algerian waste, generated in big quantity from the pastry date factories to produce a low cost adsorbent using physical activation with steam and then. We evaluated its adsorption capacity from 2,4-dichlorophenoxyacetic acid removal. To, in order to increase the adsorption capacity of the prepared activated carbon we grafted it surface by impregnation with Cobalt (Co), a transition metal; that it was played a function of catalyst support on dates pits and stems activated carbon (Co/DPSAC) to accelerate the 2,4-D adsorption rate and increase the activated carbon adsorption capacity.
Materials and Methods
Materials
2,4-Dichlorophenoxyacetic 2,4-D commercial formulation consisting of 600 g/L 2,4-D Ester butyl glycol, was used as an adsorbate in this study. 2,4-D has a chemical Formula of C8H6Cl2O3, with a molecular weight of 221.04 g/mol, and Solubility in water 900 (mg/L).
Cobalt (II) acetate tetra hydrate C4H6CoO4.4H2O with high purity was provided by (biochem/Chemapharma).
Preparation of the Activated Carbon
Dates pit and stems were obtained from the Algerian pastry date factories. First, the pits and stems were washed, and dried at 110°C for about12 hour, then, they are ground to grind between 0,5 and 4 mm and sifted. The gotten grains are impregnated in an acid sulfuric solution (H2SO4 at 60 and 40 %) respectively, washed with distilled water until neutral pH, dried at 105°C during 24 hours, and finally were kept in a desiccator to protect them from moisture. Than the precursor is pyrolysed and activated out under nitrogen flow in a horizontal tubular furnace. The reactor was a quartz tube which was placed in the furnace. For all the pyrolysis operations, about 20 g of entire date pits and stems were placed in the middle of the reactor for pyrolysis and activated for 2h under 100 cm3/min nitrogen flow and 10°C/min heating rate [11]. The pyrolysed dates pits and stems are physically activated in an inert atmosphere N2/H2O, at different temperatures (700, 800, and 900°C).
Impregnation
Dates Pits and Stems Activated Carbon (DPSAC) are first impregnated in (10 : 30) ml of ethanol/distilled water ; then we have added a solution of Cobalt (II) acetate tetra hydrate C4H6CoO4 .4H2O drop by drop at different proportion (10 wt %, 15 wt % and 20 wt %) then, pH was neutralized to 11 by drop of NaOH (1M) with magnetic stirring at room temperature for 24 h; after that the mixture was placed in ultrasonic apparatus for 2 h; finally the mixture was separated ; the obtained solid was evaporated and calcined at different temperature (400, 300 and 500°C) for 2 h .
Adsorption of 2,4-D on (DPSAC) and (Co wt%/ DPSAC)
Removal of 2,4-D from aqueous solution on DPSAC and Co/DPSAC was recording by adsorption isotherm performed in the optimal conditions.
Kinetic experiments and effects of some operating parameters (adsorbent amount, pH, Cobalt impregnation percentage, calcination temperature; and 2,4-D solution temperature) was studied.
The contact time is a very critical parameter to insure the success of the adsorbents for practical application [22-23].
Adsorption tests were performed in a set of 100 mL Erlenmeyer flasks, by contacting 50 ml 2,4-D solutions of 10 mg/L initial concentrations with 0.1g of (DPSAC) and (Cowt%/DPSAC) respectively in each flask, at pH 2, and 200 rpm.
Adsorption Isotherms
Adsorption studies were carried out in 100 ml flasks at a constant temperature (30°C), containing 0.1 g of each activated carbon and grafted adsorbent with 50 mL 2,4-D; at various initial concentrations (10, 30, 50, 80, 100, 150 mg/L). The flasks were agitated in an isothermal water-bath shaker at 200 rpm until the equilibrium was reached; the concentration of 2,4-D was determined by using UV–VIS spectrophotometer (1800 SHIMADZU) at λ max 284 nm. 2,4-D adsorption at equilibrium, qe (mg/g) was calculated by the following equation:
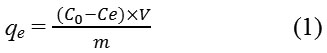
Where C0 and Ce (mg/L) are the liquid-phase concentrations of adsorbate at initial and equilibrium, respectively. V is the volume of the solutions (L), and m is the mass of the used adsorbent (g).
The adsorption removal, R (%) of 2,4-D
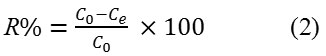
Adsorption Models
Two adsorption models (Langmuir and Freundlich) were applied to the experimental data [22, 23, 24], [25].
The linear expression for the Langmuir isotherm is
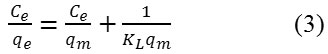
qm and KL are the maximal adsorption capacity (mg/g) and the Langmuir equilibrium constant (L/g), respectively.
The Freundlich model is given by the linear equation:
![]()
KF is the Freundlich adsorption constant (mg/g), n is a parameter usually greater than unity and related to the deviation of the isotherm from linearity. The adsorption isotherm shape depends on the n parameter value, in fact, a n higher value correspond more to the type I shape [11], [22].
Adsorption Rate Modeling
In this study, two models were used to describe the 2,4-D adsorption kinetics on the prepared adsorbent because of their simplicity and their ability to describe the adsorption kinetics of organic pollutants [9], [26,27]
The rate constant for adsorption were fitted to the pseudo-first-order and pseudo second-order kinetic equations eq. (5) and eq. (6) respectively.
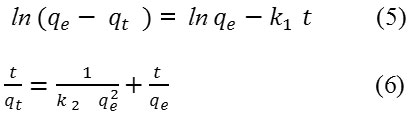
Where, qt is the amount of 2,4-D adsorbed at time, t , and k1 and k2 (minute-1) are pseudo 1st and pseudo 2nd order rate constants, respectively.
Characterization
Our raw, activated and grafted date pits and stems were analyzed using several methods.
For the thermogravimetric analysis (TGA/DTG) a thermal balance 2050 TGA V5.4A from TA instruments was used. The thermal evolution of the raw biomass and activated carbon was followed from the room temperature up to 600°C. The average mass of the sample used was of about 20 mg.
Infra-red analysis (FTIR) spectra were recorded over the wave number range 4000–650 cm−1 by using the KBr wafer technique and Perkin Elmer (Spectrum One FTIR) spectrometer.
A scanning electron microscopy (SEM) was used to observe the surface structure of raw biomass, activated and grafted materials Co20wt%/DPSAC calcined at 300°C by using High Resolution Quanta FEI200; Crech Republic.
Table 1: Nomenclature used to indicate the date pits and stems grafted under different conditions.
| Nomenclature | Indication |
| DPS | Raw dates pits and stem |
| DPSAC | Dates pits and stems activated carbon prepared at 900°C for 2h |
| Co/DPSAC | Dates pits and stems activated carbon prepared at 900°C for 2h and grafted by cobalt |
| Cowt%/DPSAC | Dates pits and stemsactivated carbon prepared at 900°C for 2h and grafted by different Co wt% |
| Co10wt%/DPSAC | Dates pits and stems activated carbon prepared at 900°C for 2h and grafted by 10 % Co |
| Co15wt%/DPSAC | Dates pits and stems activated carbon prepared at 900°C for 2h and grafted by 15 % Co |
| Co20wt%/DPSAC | Dates pits and stems activated carbon prepared at 900°C for 2h and grafted by 20 % Co |
| Co20wt% at 500°C | Dates pits and stems activated carbon prepared at 900°C for 2h and grafted by 20 % Co; calcined at 500°C for 2h |
| Co20wt% at 400°C | Dates pits and stems activated carbon prepared at 900°C for 2h and grafted by 20 % Co; calcined at 400°C for 2h |
| Co20wt% at 300°C | Dates pits and stems activated carbon prepared at 900°C for 2h and grafted by 20 % Co; calcined at 300°C for 2h |
Results and Discussion
Effect of the Activation Temperature
From (fig.1), we can observe that the adsorption capacity increase with increase of activation temperature; the best activation temperature is 900°C because the 2,4-D adsorption capacity was well. Results of (TGA/DTG) (fig.11) and the trials of 2,4-D adsorption capacity (fig.1), can confirm this fact.
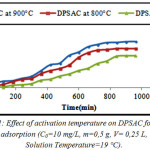 |
Figure 1: Effect of activation temperature on DPSAC for 2,4-D adsorption (C0=10 mg/L, m=0,5 g, V= 0,25 L, Solution Temperature=19°C). Click here to View figure |
Effect of the Activated Carbon Mass
The effect of different DPSAC masses (0,02-0,1- 0,2- 0,3- 0,4 and 0,5 g) on 2,4-D removal was studied. The optimal DPSAC mass was chosen when the adsorption capacity was remained constant. The 2,4-D initial concentration was fixed at 10 mg/L, the optimal mass was 0,1g as shown in (fig.2).
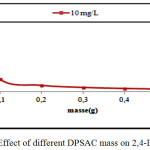 |
Figure 2: Effect of different DPSAC mass on 2,4-D removal. Click here to View figure |
Effect of Solution pH
About 0,1 g of (DPSAC) was added to 50 ml 2,4-D solution of 10 mg/l at different pH (2, 3, 5, 6, 8, 10, and 11). The pH was adjusted using 0.1 M H2SO4 and/or 0.1 M NaOH and was measured using pH meter inolab WTW PH 730. It was found that DPSAC adsorbed better the 2,4-D at pH= 2 as shown on the curve (Fig.3).
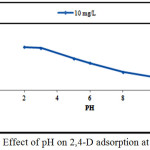 |
Figure 3: Effect of pH on 2,4-D adsorption at DPSAC. Click here to View figure |
Effect of Impregnation Proportion
The preparation of different activated carbons supported Co metal was designated as (Co/ DPSAC); (Table-1).
A series of Co/DPSAC were prepared by wetness impregnation from different content Co (10 wt%, 15 wt%, and 20 wt %) [28] .
The adsorption capacity of the three proportion prepared material were tested by 10 mg/L 2,4-D solution and compared with the DPSAC adsorption capacity. According to the adsorption tests it was found that 20 wt% of cobalt was the best percentage, the kinetics of adsorption was proportional to the higher percentage of cobalt; and the equilibrium contact time was reached at 600 min,(Fig.4).
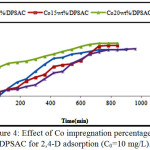 |
Figure 4: Effect of Co impregnation percentage on DPSAC for 2,4-D adsorption (C0=10 mg/L). Click here to View figure |
Effect of Calcinations Temperature on Co20wt% / DPSAC
To study the effect of calcination temperature on Co20wt%/DPSAC (prepared in the best conditions), different calcination temperature (300 °C, 400 °C and 500 °C) were used as shown in (fig.5). Results showed that the equilibrium contact time decrease with increase of calcination temperature (420 min; 600 min and 780 min). So, 300 °C is the best calcination temperature.
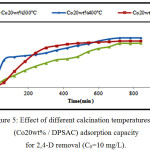 |
Figure 5: Effect of different calcination temperatures on (Co20wt% / DPSAC) adsorption capacity for 2,4-D removal (C0=10 mg/L). Click here to View figure |
Effect of 2,4-D Solution Temperature
The adsorption of 10 mg/l of 2,4-D on Co20wt% / DPSAC was tested at different 2,4-D solution temperatures (19°C; 30°C; and 60°C).
According to the equilibrium contact time of adsorption tests; it was found that the solution temperature at 30°C showed an average increase in the adsorption kinetics as exposed in (fig.6); indicating that the adsorption process of (2,4-D) on the (Co20wt%/DPSAC) is particularly exothermic.
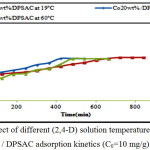 |
Figure 6: Effect of different (2,4-D) solution temperature on Co20wt% / DPSAC adsorption kinetics (C0=10 mg/g). Click here to View figure |
Effect of 2,4-D Concentration
The series of experiments were performed with different initial concentration of 2,4-D (10, 30, 50, 80, 100, and 150 mg/l); on 0,1g of Co20wt%/DPSAC calcined at 300°C.
The results (fig.7) designated that the adsorption capacity of the grafted activated carbon increases with the increase of the initial 2,4-D concentration. The adsorption kinetic was a medium and reached equilibrium between 300 min and 540 min, with maximum adsorbed amount of 31 mg/g.
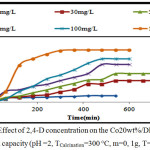 |
Figure 7: Effect of 2,4-D concentration on the Co20 wt% / DPSAC adsorption capacity (pH =2, TCalcination=300 °C, m=0, 1g, T=30°C) Click here to View figure |
Effect of Contact time on the Adsorption Removal
The effect of contact time on the amount of 2,4-D adsorbed by (DPSAC) and (Co20wt%/DPSAC) was studied using 10 mg/l as an initial concentration of (2,4-D) solution; The results showed that the adsorption removal of the grafted activated carbon Co20wt%/DPSAC calculated from eq.(2) was 95.02% at 300 min and 90,60% at 800 min for activated carbon (DPSAC),(Fig.8).
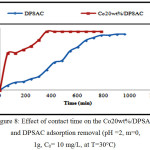 |
Figure 8: Effect of contact time on the Co20wt%/DPSAC and DPSAC adsorption removal (pH =2, m=0, 1g, C0= 10 mg/L, at T=30°C) Click here to View figure |
Adsorption Isotherms
Adsorption isotherms of 2,4-D on the activated carbon (DPSAC) and grafted activated carbon (Co20wt%/DPSAC) are reported in (Fig.9). We observe that the isotherms were of type I; Maximal 2,4-D adsorption capacity definite at the plateau of the isotherm are 29,85 and 32,50 mg/g for (DPSAC) and (Co20wt%/DPSAC) respectively.
The equilibrium adsorption data were interpreted using Langmuir and Freundlich models (Fig.9) ; Values of Langmuir and Freundlich parameters are given in Table 2.
from Table–2, we can observe that grafted activated carbon have advanced adsorption capacity and it have accelerated the adsorption reaction compared with the activated carbon, tested for pesticides acid 2,4-D adsorption ;
(qe (DPSAC) qe(Co20wt%/DPSAC)). (Fig.9) illustrate that the fit between experiments and Langmuir model is adequate. The comparison of correlation coefficients (R) of the linearized form of both equations indicates that Langmuir equation better represent the data than Freundlich equation for the experimental equilibrium adsorption data. This indicates that the monolayer adsorption plays a result role in 2,4-D removal from aqueous solution.
The equilibrium parameter (RL) [29, 30], is the important parameter characteristic of the Langmuir isotherm. The parameter is defined by eq. (7)

Where KL is the Langmuir constant and C0 (mg/L) is the highest initial 2,4-D concentration. The parameter RL indicates the shape of isotherm as follows [29];
Table 2.a
| Value of RL | Type of isotherm |
| RL>1 | Unfavorable |
| RL=1 | Linear |
| 0<RL<1 | Favorable |
| RL=0 | Irreversible |
Value of RL was established to be 0,066 and 0,031 at T=30 °C. This again confirmed that the Langmuir isotherm was favorable for adsorption of 2,4-D on the (DPSAC) and (Co20wt%/DPSAC) respectively used in this study.
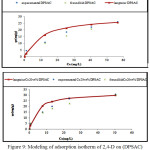 |
Figure 9: Modeling of adsorption isotherm of 2,4-D on (DPSAC) and (Co20 w%t/DPSAC, Tcalcination =300°C), (at T=30°C) Click here to View figure |
Table 2: Langmuir and Freundlich parameters for the adsorption of 2,4-D in aqueous solution on (DPSAC) and (Co20w%t/DPSAC)
| Models parameters | DPSAC | Co20wt%/DPSAC) Tcalcination =300°C |
| Langmuirqm (mg/g)KL (L/mg)R | 29,850,0900,976 | 32,500,2240,996 |
| FreundlichKF (mg/g)nR | 3,29391,68600,950 | 6,87232,62950,9803 |
Adsorption Rate
As qe evaluated from fitting experimental data to Eq. (6) closely coordinated (3,9872 mg/g and 4,7348 mg/g) for (DPSAC) and (Co20wt%/DPSAC) at 30°C with the experimental values (4.535 mg/g and 4,775 mg/g ) for (DPSAC),(Co20wt%/DPSAC) respectively at 30°C ; 2,4-D adsorption shown to have been well modelled by pseudo-second-order kinetics model (Fig.10 (a) and (b)). It is apparent from the results listed in Table-3 that 2,4-D adsorption rate for (Co20wt%/DPSAC) was higher than that for (DPSAC).
 |
Figure 10: Pseudo first order and pseudo second order models for adsorption of 2,4-D by DPSAC fig (a), fig(b) by (Co20wt%/DPSAC, Tcalcination =300°C ) at 30°C. Click here to View figure |
Table 3: Rate kinetics parameters
| Models | Parameters | DPSAC | Co20wt%/DPSAC |
| (Pseudo-first-order) | K1qeR | 0,00192,36740,9514 | 0,00422,57330,9730 |
| (Pseudo-second-order) | K2qeR | 0,00383,98720,9889 | 0,0044,73480,9921 |
Characterization
Thermo Gravimetric Analyses
The (TGA/DTG) thermal curve of raw date pits and stems (DPSAC) are exposed in (fig.11).TGA analyses demonstrated that the process of mass loss contained 5 sections. First, the mass loss 6,031% resulting in moisture elimination occur at 65,54°C, in the second phase, the process of decomposition attains 39.15% load loss at the maximum rate of 278,69°C. The third stage was found at 328,90°C with load loss of 12,48%. After that at 365,25°C there is a loss of 7,709% of mass, the last mass loss was 6,055%, it occurs at 409,35°C.
At first, hemicelluloses decompose between (200 and 300°C), next cellulose (300 and 380°C) and finally lignin at (180–500°C) [22], [6], [31].
 |
Figure 11: TGA/DTG analysis of raw dates pits and stems (DPS). Click here to View figure |
The (TGA/DTG) thermal curve of date pits and stems activated carbon (DPSAC) are shown in (fig.12).
This figure illustrates that the process of mass loss contained 2 sections. First the mass loss 15,18% resulting in moisture elimination come about 51°C. The second it remained constant up to 500°C. After 500°C there is a degradation of 81,76 % of the activated carbon at 900°C during 2 h.
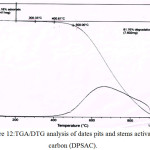 |
Figure 12: TGA/DTG analysis of dates pits and stems activated carbon (DPSAC). Click here to View figure |
The FTIR Analysis
The FTIR spectra of raw activated and cobalt grafted date pits and stems are shown in (fig.13).
Spectra of dates pits and stems shows many bands characteristic of cellulose, hemicelluloses and lignin, the main compounds of dates pits and stems. Peaks at 3726-3629 and 3400 cm-1 are attributed to O-H stretching vibration in hydroxyl groups [32]. The bands observed at 2923, 2852 and 2360 cm-1 are assigned to asymmetric and symmetric C-H stretching in alkyl groups and C=C groups respectively [32, 33].
C=O stretching in aldehydes, ketons groups and esters are observed at 1740 cm-1 while the skeletal (C=C) stretching in aromatic rings are observed at 1435 cm-1. The bands at 1365, 1228 and 719 cm-1 are assigned to (C-H) deformations in lignin and (C-H) deformation in cellulose [34].
After the thermal activation of dates pits and stems, the peaks between 3726 and 2852 cm-1 exhibit a lower intensity than the raw state because of degradation of water and aliphatic compounds of dates pits & stems. New band assigned to C=C skeletal stretch in condensed aromatic system appeared at 1550 cm-1 indicating the increase of aromaticity in the obtained materials.
The same peaks are observed in FTIR spectra of grafted date pits and stems with cobalt. However a new peaks characteristic of Co/ (DPSAC) appeared after impregnation. The peak initially observed at 1555 cm-1 was shifted to 1549 cm-1 in the grafted cobalt date pits and stems activated carbon, and a new peak appears at 1014 cm-1 probably due to the interaction of CO3O4 on grafted cobalt date pits and stems activated Co/(DPSAC). The similar peaks were observed in other study [35].
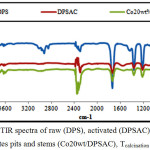 |
Figure 13: FTIR spectra of raw (DPS), activated (DPSAC) and cobalt treated dates pits and stems (Co20wt/DPSAC), Tcalcination = 300°C. Click here to View figure |
IR Spectra obtained for date pits and stems grafted with different cobalt percentage (10wt%, 15wt%, and 20 wt %); and different calcinations temperatures (300, 400, and 500°C) as shown in (Fig.14) and (Fig.15) respectively, exhibit a similar shape, wish indicate that the same functional groups are present in the materials.
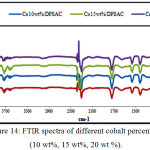 |
Figure 14: FTIR spectra of different cobalt percentage (10 wt%, 15 wt%, 20 wt %). Click here to View figure |
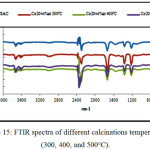 |
Figure 15: FTIR spectra of different calcinations temperatures (300, 400, and 500°C). Click here to View figure |
Scanning Electron Microscopy (SEM)
Scanning electron microscopy (SEM) of raw activated and cobalt grafted date pits and stems are shown in Fig.16 (A, B, C). It can be seen from the (Fig.16 (A)) that the raw date pits and stems have irregular and plugged porous structure but after activation (Fig.16 (B)) a well degaged and regular porous structure is created. The development of porosity is attributed to the removal of volatile matters resulting from the decomposition of major compounds of dates pits and stems (cellulose- hemicelluloses and lignin) at high temperature. (Fig.16(C)) illustrates the surface morphology of Co/DPSAC. We can observe that the surface became porous and smooth; this may be due to the deposition of cobalt at the surface of the material (DPSAC).
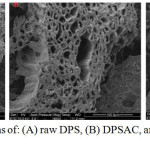 |
Figure 16: SEM micrographs of: (A) raw DPS, (B) DPSAC, and (C) Co20wt/DPSAC, Tcalcination =300°C. Click here to View figure |
Conclusion
Pyrolysis and activation after impregnation of date pits and stems with different cobalt contents are investigated for their potential to accelerate reaction of pesticide acid 2,4-D adsorption from aqueous solution. the material (Co20wt%/DPSAC), was prepared in the optimum conditions : first DPSAC is prepared by pyrolysis and activation at 900°C for 2h, then grafted by 20 wt % of Co; and calcined at 300 °C for 2h. The results shows that the grafted material with Cobalt can play also a roll of catalyst and it can enhance the adsorption kinetics of 2,4-D acid in aqueous solution. In fact, the adsorption of 2,4-D on (Co20wt%/DPSAC) was faster, than this one on (DPSAC); the time necessary to attain adsorption equilibrium was between 300 min and 540 min for (Co20wt%/DPSAC) and between (800 min to 1440 min) for (DPSAC) respectively. Also, the maximum adsorption capacity was higher (32,50 mg/g) for (Co20wt%/DPSAC) than the other one (29, 85 mg/g) in (DPSAC). Adsorption data can be effectively modeled by Langmuir model. The adsorption of 2,4-D on (DPSAC) and (Co20wt/DPSAC) obeys the second-order model.
The characterization of the raw, the activated carbon and the grafted material by TGA/DTG showed that 900 °C and 300 °C is the pyrolysis and activation temperatures for Dates pits and stems (DPS) and Co/DPSAC degradation. FTIR has been shown the Location of active phase into the surface of DPSAC to form Co3O4. SEM micrographs show that the surface became porous and smooth after impregnation with Cobalt. Cobalt grafted dates pits and stems activated carbon was found to be best effective and fast in pesticide acid 2,4-D removing from contaminated water.
References
- Yu,J.G.; Zhao,X,H ;Yang ,H.; Chen, X.H; Yang, Q.; Yu, L.Y.; Jiang, J.H.; Chen, X.Q. Science of the Total Environment. 2014, 482–483,241–251
CrossRef - Volodymyr, I.; Lushchaka.environnemental toxicology and pharmacology .2014, 37,861–869
- Jhung, S. H.; Chemical Engineering Journal. 2013, 234, 99 –105
CrossRef - Yang, Q.; Chen, H.; Zhang, Z.; Feng, M.; Liu, W.; Wan, W. Chemical Engineering Journal.2017, 313,498–507
CrossRef - Ali, I.; Asim, M.; Tabrez A. Kh. Journal of Environmental Management. 2012,113, 170-183
CrossRef - Hadoun, H.; Sadaoui, Z. ; Souami, N. ; Sahel, D. ; Toumert, I. Applied Surface Science .2013, 280,1– 7
CrossRef - Duran-Valle, J.C.; Gomez-Corzo, M.; Pastor-Villegas, J. V. ; Gomez-Serrano, J. Anal. Appl. Pyrolysis .2005, 73, 59–67.
CrossRef - Tseng, R.L.; Tseng, S.K. Journal of Colloid and Interface Science.2005, 287
- Djilani.; Zaghdoudi, R.; Modarressid, A.; Rogalski, M.; Djazi. F; Lallam.A. Chemical Engineering Journal.2012, 189– 190,203– 212.
- Sych, N.V.; Trofymenko, S.I.; Poddubnaya, O.I.; Tsyba,M.M.; Sapsay, V.I.; Klymchuk, D.O. ; Puziy, A.M. Applied Surface Science .2012 , 261 ,75–82.
CrossRef - Bouchelta, C.; Medjram, M. S. ; Marsa. ; Ahmed Chekkat, F. ; Ramdane, N. ; Bellat, J.P .Journal of Analytical and Applied Pyrolysis .2012,94,215–222
CrossRef - Kus´mierek, K. ; wia˛ tkowski ,A. S´. Water Environ. Res.2016,88
- Gupta, V.K. ; Ali, I. ; Saini ,V.K. J. Colloid Interface Science.2006, 299,556–563
CrossRef - Sachin ,A. M.; Nikhilesh, S. T.; Rhushikesh, A. Kh. Arabian Journal of Chemistry. 2016.
- Deng, S.; Ma, R.; Yu, Q.; Huang, J.; Yu, G. J. Hazard. Mater.2009, 165,408-414.
- Hameed, B.H.; V. O. Njoku, Azharul Islam, Md.; Asif, M. Journal of Environmental Management.2015, 154,138-144.
CrossRef - Hameed, B.H. ; Salman, J.M. ; Njoku,.V.O. Chemical Engineering Journal .2011,174,41– 48
CrossRef - Hameed, B.H. ; Salman, J.M. ; Njoku,.V.O. Chemical Engineering Journal .2011,174,33– 40
CrossRef - Dehghani, M. ; Nasseri. S. Karamimanesh.. J. Environ. Health Sci. Eng .2014,2,28-38.
CrossRef - Roy, D.; Manna, S.; Saha, P.; Sen, R.; Adhikari. B. Journal of the Taiwan Institute of Chemical Engineers.2016, 67,292–299.
- Yu, Y.; Zhang, Ch.; Yang, L.; Chen, J. P.Chemical Engineering Journal 2016.
- Mechati, F.; Bouchelta, C.; Medjram, M.S.; Benrabaa, R.; Ammouchi, N. Journal of Environmental Chemical Engineering.2015, 3, 1928–1938.
CrossRef - Ahmed, M.J. ; Theydan,S.K. J. Anal. Appl. Pyrol. 2014, 105,199–208, doi:http://dx.doi.org/ 10.1016/j.jaap.2013.11.005.
CrossRef - Foo, K.Y.; Hameed, B.H. Desalination. (1–3) 2011, 275,302–305.
CrossRef - Do, D. D. Adsorption Analysis: Equilibria and Kinetics. Imperial College Press, London, 1998.
- Escudero, C.; Gabaldon, C.; Marzal, P.; Villaescusa, I. J. Hazard. Mater, 2008, 152 , 476–485.
CrossRef - Azouaou, N. ; Sadaoui, Z. ; Djaafri, A. ; Mokaddem, H. J. Hazard. Mater, 2010, 184,126–134.
CrossRef - Zhang, G.; Su, A. ; Du, Y. ; Qu, J. ; Xu, Y. Journal of Colloid and Interface Science .2014, 433,149–155.
CrossRef - Hameed, B.H.; Shaarani, F.W.,Desalination, . 2010. 255, 159–164.
CrossRef - Weber, T.W.; Chakkravorti, R.K. AIChE J. 1974, 20,228–238.
CrossRef - Uysal, T. ; Duman, G.; Onal, Y.; Yasa, I.; Yanik. J. Anal. Appl. Pyrol.2014, 108, 47-55.
CrossRef - Bouchelta, C. ; Medjram, M.S. ; Bertrand, O. ; Bellat, J.P. J. Anal. Appl. Pyrolysis.2013, 82,70–77.
CrossRef - Jeguirim, M.; Belala, Z.; Addoun, F. Gwenaëlle Trouve. Desalination. , 2011, 271, 80–87.
- Muthanna, A.J.; Asmaa, A.F. Journal of Water Process Engineering. , 2016, 9,201–207.
CrossRef - Karthikeyan, S.; Boopathy, R.; Sekaran, G. J. Colloid Interface Sci., 2015, 001125, S0021-9797.

This work is licensed under a Creative Commons Attribution 4.0 International License.









