Two New Bioactive Biphenylpropanoidsfrom the Roots of Salsolaimbricata (Chenopodiaceae) Growing in Saudi Arabia
Mohamed Habid Oueslati1,2, Jalloul Bouajila3 and Hichem Ben Jannet4
1Northern Border University, College of Science, Department of Chemistry , P.O. Box 1231, Arar 91431, Kingdom of Saudi Arabia.
2Carthage University, Department of Chemistry, Preparatory Institute for Scientific and Technical Studies, P.O. Box 51, La Marsa 2070, Tunisia.
3Laboratoire des IMRCP UMR CNRS 5623, Faculté de Pharmacie de Toulouse, Université de Toulouse, Université Paul-Sabatier, 118 Route de Narbonne, Toulouse F-31062, France.
4Laboratoire de Chimie hétérocyclique, Produits Naturels et Réactivité Faculté des Sciences de Monastir, Université de Monastir, Monastir 5019, Tunisia.
Corresponding Author E-mail: oueshabib@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/330432
Phytochemical investigation of the roots of Salsolaimbricata allowed to two new bioactive biphenylpropanoids(1) and (2) named biphenylsalsonoidA and B, respectively. Their structures were established through spectroscopic methods (1D and 2D NMR, (ES)-HRMS, and IR).The isolates were tested for their antioxidant activity using DPPH● and ABTS●+ assays. 1 and 2 showed a moderate activity towards DPPH (IC50 = 86.5 ± 1.3 and 122.3 ± 0.63 μg/mL, respectively) and ABTS (IC50 = 95 ± 1.5, 137.7 ± 1.2 μg/mL, respectively). The antibacterial effect of the ethyl acetate extract and the isolates were assessed. Resultsobtained revealed that compounds showed important antibacterial activities against S. aureus, S. epidermidis, M. luteus, and E. coli with MIC values ranging from 16 to 32 μg/mL.
KEYWORDS:Salsolaimbricata; biphenylsalsonoids; NMR; antioxidant activities; antibacterial activities
Download this article as:| Copy the following to cite this article: Oueslati M. H, Bouajila J, Jannet H. B. Two New Bioactive Biphenylpropanoidsfrom the Roots of Salsolaimbricata (Chenopodiaceae) Growing in Saudi Arabia. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Oueslati M. H, Bouajila J, Jannet H. B. Two New Bioactive Biphenylpropanoidsfrom the Roots of Salsolaimbricata (Chenopodiaceae) Growing in Saudi Arabia. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35495 |
Introduction
The genus Salsolaincludes halophyte species and belongs to the family of Chenopodiaceae1-3. The genus is widespread in the dry regions of Middle East, Africa, and Europe. Many species among the genus are used in traditional medicine. In the Middle East, Salsolabaryosmais used as a diuretic agent and against some inflammations 4. This plant also exhibits antioxidant activities5, alkaloids (salsolin and salsolidin) have been isolated from Salsola tragus (synonym: Salsolakali) used in the treatment of hypertension by stimulating the activity of sleep6,7. At present, only few species of the genus have been studied chemically and biologically and were found to be a good source of phenolic compounds identified in S. kali, S. soda, S. oppositifolia and S. collina8,9. Furthermore, antioxidant triterpeneswere isolated from S. baryosma and S.somalensis10 and new antioxidant bibenzyl derivative and isoflavonoidwere isolated from S. tetrandra11. Our previous work on the genus led to salsolanol and biphenylsalsinolisolation from S. villosa12 and cleomiscosin D, norisoprenoid, long-chain hydroxyl fatty acids, taxiphyllin, trans-N-feruloyltyramineS-(-)-trans-N-feruloyloctopamine and coumarinolignan from S. tetrandra13,14.Recent research on Salsolaimbricata showed the presence of triterpenesaponins from the methanolic extract of roots 14 and new isorhamnetin derivatives from theleaves15. Thus, in order to continue ourresearch on the genus of salsolagrowing in Saudi Arabia.We focused our study on the ethyl acetate extract from the roots of S. imbricata because it has not been studied previously. Indeed, the present study suggests isolating new compounds with important biological activities. We try to the isolation of new bioactivecompounds from the roots of S. imbricate and the evaluation of its antibacterial activity against Gram-positive and Gram-negative bacteria. Furthermore, the in vitro antioxidant activity was tested by using DPPH● and ABTS●+ assays of isolated compounds.
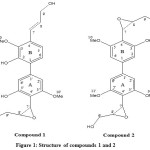 |
Figure 1: Structure of compounds 1 and 2 |
Materials and Methods
General Procedures
Theoptical rotations were recorded on a Perkin-Elmer 241-MC polarimeter. UV spectra were measured by using a Shimadzu UV-2401A spectrophotometer. Infra-red spectra were measured on a Perkin-Elmer 157G. 1H, 13C and 2D NMR spectra of isolated1 and 2 were obtained inCD3OD on Bruker)300 MHz, 75 MHz(spectrometer using internal reference the residual solvent resonance. Coupling constants were measured in Hertz and chemical shifts were reported in ppm. ESI-HRMS was measured on a Shimadzu LC-MSSpectrometer.
Plant Material
Roots of SalsolaimbricataDelile. ex Schul. Were collected from Arar, Saudi Arabia, on November 2015. The plant was identified by Dr. Ahmed K. Osman, College of Sciences, Department of Biology, Kingdom of Saudi Arabia and deposited in the herbarium (Sv-26) of the above department.
Extraction and Isolation
The roots from S. imbricata were dried and then ground into powder. 1Kg of powder was extracted with methanol (5L). After that the crude extractwas evaporated in vacuoyielding a residue of 65.4g (%).The residue was dissolved in water (2 L) and then extracted successively with petroleum ether, ethyl acetate and n-butanol yielding 12.4, 16.2 and 24.6 g sub-extracts, respectively. The ethyl acetate extract was fractionated on a column chromatography(silica gel- mesh 70-230, 70 × 5 cm, i.d.)eluted with mobile phase of n-hexane/EtOAc (100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100, 1L each) and EtOAc/MeOH (90/10 80:20, 70:30, 60:40, 50:50, 500 mL each ). After TLC monitoring the column chromatographic fractions were combined into eight fractions (F1– F8). Fraction F4 (426 mg) was separated on a silica gel column (mesh 70-230, 70 × 2 cm, i.d) that was eluted with CHCl3/MeOH(100:0 to 60:40) to obtained four subfractions (A1to A4). The purification of the fractionof the subfractionA2 (62mg) by using preparative TLC 85:15 (CHCl3/MeOH) to yield 16 mg of 1 and 12 mg of 2.
Antibacterial Activities
The antibacterial activities were tested against Gram+ (Staphylococcus aureus, S. epidermidis and Micrococcus luteus) and Gram-negative strains (Pseudomonas aeruginosa, Escherichia coli, and Salmonella typhimurium). The tested extracts were respectively: Methanol crude of the roots, the isolated compound 1 and the isolated compound 2. Mueller-Hinton agar (5ml) was used for the culture of bacteria (stored at -70°C stock) and the media were incubated for 24 h at 37°C.
The antibacterial activity was evaluated by minimum inhibitory concentration (MIC)16,17. Serial tube dilution was used to determine the values of MIC for the methanol crude extract and for the two isolated compounds. To obtain stock solution, 0.5 mg of plant extracts (methanol crude, compound 1 and 2) was suspended in 2 mL of distilled water and 2 tween-80 drops for the homogenization. The suspensions of micro-organisms consist of a medium with the concentration fixed at 107 organisms/mL and one drop of suspension (0.02 mL) was added to the broth dilution. The temperature of the incubation was fixed at 37°C for 18 hours and the tubes were examined for the growth. The MIC of the tested extract/products was fixed for the lowest concentration that showed the totally absence of the growth for the micro-organisms. The negative and the positive control consist, respectively, of distilled water with 2 drops of tween-80 and kanamycin.
Antioxidant Activities
Free Radical Scavenging Ability using DPPH● Radical
The protocol was used as described, previously, by Tepe, B.et al. 200519. Briefly, 2 mL of DPPH solution (100 μg/mL, EtOH) was added to 0.5 mL of compounds (0.01–1 mg/mL). After 30 min, the absorbance was read at 517 nm. The blank consists of 2 mL of DPPH solutionand 0.5 mL of methanol. The IC50 was determined by the following equation:
%Inhibition = [(Ablank – Asample)/Ablank)] x 100
Ablankand Asamplewere, respectively, the absorbance values of the control and the test sample. Vitamin C was used as reference and tests were measured in triplicate.
Free Radical Scavenging Ability using ABTS●+ Radical Cation
ABTS was dissolved in distilled water and the concentration was fixed at 7 mmol/L20. For the completion of radical generation, ABTS●+radical cation was generated by adding the potassium persulfate (2.45 mmol/L) to the ABTS solution. The mixture was conserved in the darkness for 12–16 h at room temperature. After a dilution of the mixture with ethanol, the wavelength was fixed at 734 nm until to obtain an absorbance value of 0.70 ± 0.02. ABTS solution (50 mL)was added to 950 mL of compounds (0.01–1 mg/mL) and after 6 min the absorbance was measured at 734 nm. The blank consists of 50 mL of ABTS solution and 950 mL of ethanol. The IC50 was determined by the formula mentioned before in DPPH assays.
Results and Discussion
Compound 1 (Fig. 1) has a molecular formula of C20H22O7 as deducted from the ESI-HRMS (m/z = m/z 397.1260 [M+Na]+). The IR spectrum revealed the presenceofhydroxylgroup (3446 cm-1) and aromatic ring (1625 cm-1).
In the aromatic region of the spectrum 1H NMR of 1 (Table 1) displayed proton signals at δH 6.98 (H-2’, d, J= 1.9Hz) and 6.84 (H-6’, d, J= 1.9Hz), attributableto the meta-coupled protons, of the terasubstituted aromatic ring A and two aromatic proton signals at δ H 7.01 (H-5, d, J=8.1Hz) and 6.78 (H-6, d, J= 8.1Hz) attributable to the two ortho-coupled protons of the tetrasubstituted aromatic ring B. The same spectrum displayed the presence of two trans-olefinic protons resonating at δH5.55 (1H, d, J=15.9Hz) and at δH6.25 (1H, dt, J=15.9Hz, J= 6.0Hz), attributable to H-7 and H-8, respectively, as well as two methoxy groups at δH 3.76 (3H, s) and 3.84 (3H, s) assignable to H-10 and H-10’, respectively.
The 13C-NMR and DEPT spectra of 1 showed signals for 14 sp2 carbons (seven methines and seven quaternary carbons) and 6sp3 carbons (two methyl, two methylene and two methine groups) (Table 1).
The full analysis of the 1H and 13C NMR spectra were obtained by using 2D NMR. The correlations observed in the 1H-1H COSY spectrum between the olefinic protons (H-7 and H-8) and the hydroxymethylenic protons (H-9) provided evidence for the propenol moiety (-CH=CH-CH2OH). The 2J and 3J correlation of the olefinic proton H-7 (δH 6.25) with C-3 (δC 151.2), C-4 (δC 126.4), and C-5 (δC 112.5)revealed in the HMBC spectrum (Fig. 2) showed that the propenol moiety is connected to the ring B at C-4 (Fig. 2). This position was consolidated by the appearance of the noeH-7/H-10(OMe). The detection of signals at δH/ δC5.52/ 87.7 and 3.42 / 56.3 suggested the presence of a 1,2-disubstituted epoxide in the molecule 12,21,22. The correlations H-7’/H-8’ and H-8’/H-9’ observed in 1H-1H COSY spectrum (Fig. 2) indicated an epoxy substituted propanoid moiety. HMBC cross-peaks of C-4’(δC 126.3), C-3’(δC 149.5), and C-5’(δC 150.1) with H-7’ (δH5.52) confirmed the attachment of this epoxy propanoid moiety to the trisubstituted aromatic ring A at C-4’ (Fig. 2). This result was consolidated by the neo between H-7’ and methoxy H-10’ revealed in the NOESY spectrum (Fig. 2). The biphenylic structure of compound 23-24 and the connection of two ring A and B at C-1 and C-1’ were evidenced by the 3J correlations H-6/C-1’ and H-2’/C-1 observed in HMBC spectrum(Fig. 2). The position of the two methoxy groups was confirmed by the 3J correlations H-10 (OMe)/C-3 and H-10’(OMe)/C-3’ in the HMBC spectrum (Fig. 2) and by the appearance of the noe cross peaks H-7/ H-10(OMe) and H-7’/H-10’(OMe) in the NOESY spectrum. The above data, were found to be consistent with a new biphenylpropanoid structure identified to be 4′-(9′- (hydroxymethyl) oxiran-7′-yl)-4-((E)-3-hydroxyprop-7-en-7-yl)-3,3’-dimethoxy-[1,1′-biphenyl]-2,5’-diol named biphenylsalsonoid A
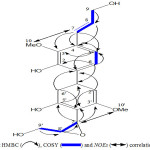 |
Figure 2: Relevant HMBC (→), COSY (=) and NOEs (↔) Correlations for compound 1 |
Compound 2 (Fig. 1) Fig. 1) has a molecular formula of C21H24O8as deducted from the ESI-HRMS (m/z = m/z 427.1365[M+Na]+). The IR spectrum revealed the presenceofhydroxylgroup (3448 cm-1) and aromatic ring (1622 cm-1).
The aromatic region of the 1H-NMR 2(table 1) spectrum showed characteristic singlets at δH 6.58 (2H, s) which were attributed to the equivalent protons H-2’ and H-6’ of the tetrasubstituted aromatic ring A. The same region exhibited two aromatic signals at δH 6.87 (d, J= 1.8Hz) and at δH 7.01 (d, J= 1.8Hz) attributed to H-2 and H-6. In addition, the spectrum showed a singletat δH 3.78 (6H, s) attributed to the two equivalent methoxygoups H-10’and H-11’ (-OCH3) attached to the aromatic ring A and another singlet at δH 3.76 (3H, s) corresponding to the methoxy group H-10 (-OCH3) attached to the second aromatic ring B. The 1H-NMR,13C-NMR and HMQC spectra exhibitedcharacteristic resonances of two disubstituted epoxides12,21,22. at δH/ δC 5.51 (H-7, m)/ 87.3 (C-7), 3.42 (H-8, m)/ 56.2 (C-8), 5.46 (H-7’, m)/ 87.1(C-7’) and 3.40 (H-8’, m)/ 56.0 (C-8’).
The 13C spectrum of 2 showed resonance of 12 sp2 carbons attributable to eight quaternary carbons which four are oxygenated and four tertiary carbons (Table 1). The same spectrum also showed three methoxy carbons at δC 56.8 and six sp3 oxygenatedcarbons in the region δC 56.0-87.3 (Table 1).
The 1H-1H COSY experiment (Fig. 3) showed correlations H-8 with H-7andH-9on the one hand and /H-8’ with H-7’andH-9’on the other hand provided evidence for the two epoxy propanoid moieties. The above spectral data indicate that 2 and 1 are two analogous compounds.
The HMBC long-range 2J and 3Jcorrelations H-2with C-1, C-3 and C-6; H-6with C-4; and H-2’with C-1, C-3’ and C-4’ indicated 2 should be processed a biphenyl skeleton23,24 (Fig. 3). The location of the hydroxyl function, the three methoxy groups and the two epoxy propanoids one the biphenyl skeleton were evidenced by the HMBC and the NOESY experiments. The presence of the first epoxy propanoid at C-4 was established by the correlations of the proton H-7 (δH 5.51) with C-4 (δC 126.8), C-3 (150.1) and C-5 (152.4) observed in HMBC spectrum. The location of the methoxy group at C-5 was confirmed by the 3 J correlations H-10/C-5 deduced from the HMBC spectrum (Fig. 3) and by the appearance of the noe between H-7 and H-10(-OMe)observed in the NOESY spectrum. The second epoxy propanoid at C-4’ was clearly indicated by the correlations of the proton H-7’ (δH 5.46) with (C-3’and C-5’, δC 152.2) and C-4’ (δC 126.2) observed in HMBC spectrum (fig 3). The position of the two equivalent methoxy groups at C-3’ and C-5’ was established by the 3JC-H correlationsof the protons resonating at δH 3.78 (H-10’,11’) and the aromatic quaternary carbons C-3’, C-5’ (δC 152.2) (Fig. 3). This result was reinforced by the noe cross peak between the proton H-7’ (δH 5.46) and the equivalent protons of the two methoxy groups H-10’ and H-11’ (δH 3.78) (Fig. 3). The above data, were found to be consistent with a new biphenylpropanoid structure identified to be 4,4′-bis-(9-hydroxymethyl) oxiran-7-yl)-5,3′,5′-trimethoxy [1,1’biphenyl]-3-ol named biphenylsalsonoid B.
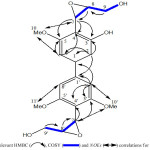 |
Figure 3: Relevant HMBC (→), COSY (=) and NOEs (↔) correlations for compound 2 |
Table 1: NMR spectral data of compounds 1 and 2 (CD3OD, 300 MHz, J in Hz).
|
|
Compound 1 |
Compound 2 |
|
||
|
Position |
13C(δ) |
1H(δ) |
13C(δ) |
1H(δ) | |
|
1 |
139.5 |
– |
138.7 |
– | |
|
2 |
149.0 |
– |
112.4 |
7.01(1H, d, J=1.8) | |
|
3 |
151.2 |
– |
150.1 |
– | |
|
4 |
126.4 |
– |
126.8 |
– | |
|
5 |
112.5 |
7.01 (1H, d, J=8.1) |
152.4 |
– | |
|
6 |
116.7 |
6.78 (1H, d, J= 8.1) |
110.2 |
6.87 (1H, d, J=1.8) | |
|
7 |
132.5 |
6.25 (1H, d, J=15.9) |
87.3 |
5.51 (1H, m) | |
|
8 |
127.9 |
5.55 (1H, dt, J=15.9 J= 6.0) |
56.2 |
3.42 (1H, m) | |
|
9 |
64.5 |
4.21 (2H, d, J= 5.7) |
64.6 |
4.32 (1H, m) | |
|
10(OMe) |
56.4 |
3.84 (3H, s) |
57.2 |
3.76 (3H, s) | |
|
1’ |
136.0 |
– |
138.4 |
– | |
|
2’ |
113.9 |
6.98 (1H, d, J= 1.9) |
108.6 |
6.58 (1H, s) | |
|
3’ |
149.5 |
– |
152.2 |
– | |
|
4’ |
126.3 |
– |
126.2 |
– | |
|
5’ |
150.1 |
– |
152.2 |
– | |
|
6’ |
110.4 |
6.84 (1H, J= 1.8) |
108.6 |
6.58 (1H, s) | |
|
7’ |
87.4 |
5.52 (1H, d, J= 6.3) |
87.1 |
5.46 (1H, m) | |
|
8’ |
56.3 |
3.42 (1H, m) |
56.0 |
3.40 (1H, m) | |
|
9’ |
64.3 |
4.31 (2H, m) |
64.4 |
4.32 (2H, m) | |
|
10’ (OMe) |
57.2 |
3.76 (3H, s) |
56.8 |
3.78 (3H, s) | |
|
11’( OMe) |
– |
– |
56.8 |
3.78 (3H, s) | |
Antioxidant Activities
For the antioxidant activities of the isolated compounds 1 and 2, two assays have been assessed: DPPH free radical scavenging and ABTS system (Table 2).
The isolate biphenylpropanoids 1 and 2 showed a moderate antioxidant activity towards DPPH with IC50 values of 86.5 ± 1.3 and 122.3 ± 0.63 μg/mL, respectively, but less potent when compared to Vitamin C. On the other hand, the isolated biphenylpropanoids showed antioxidant activity against ABTS in the similarly order as against DPPH (IC50 = 95 ± 1.5, 137.7 ± 1.2 μg/mL, respectively). Compound 1 has a relatively high activity due to thepresence of two phenol groups by comparison with 2 bearing one phenol group.
Table 2: Antioxidant (DPPH● and ABTS●+ Assays) activities of compounds 1 and 2
|
IC50 values (μg/mL) |
|||
|
1 |
2 |
Vitamin C |
|
| DPPH● |
86.5 ± 1.3
|
122.3 ± 1.4 |
26.0 ± 1.2 |
| ABTS●+ |
95.1 ± 1.5
|
137.7 ± 1.2 |
22.4 ± 0.5 |
Antibacterial Activities
The in vitro antibacterial activity of the EtOAc extract of the roots of S. villosa,compounds 1 and compound 2 was assessed using the MIC method against three Gram-positive bacteria; S. aureus, S. epidermidisand Micrococcus luteusand three Gram-negative bacteria; Escherichia coli, Pseudomonas aeruginosaand Salmonella typhimurium.
According to the results given in Table 3. The compounds 1 and 2 display the same activity against the three Gram-negative used and the Gram-positive strains S. aureus and S. epidermidis. This finding could be due to the common biphenyl skeleton bearing in the same position (C-4′) present in both 1 and 2. On the other hand, Compound 2 (MIC = 16 μg/mL) was found to be two times more active than compound 1 against M. luteus. This dual sensitivity against the compound 2 might be explained by the difference in structure between the two compounds, mainly the apparition of a new epoxy moietyat C-4 and another methoxy group at C-5′.In addition to the modification in the position of the hydroxyl group by comparison to compound 1.
Table 3: Antibacterial activities of EtOAc extract and compounds 1 and 2
| Strains |
MIC values (μg/mL) |
|||
|
EtOAc Extract |
1 |
2 |
Kanamycin |
|
| Gram-positive | ||||
| S. epidermidis |
32 |
32 |
32 |
2 |
| S. aureus |
16 |
16 |
16 |
2 |
| M.s luteus |
16 |
32 |
16 |
4 |
| Gram-negative | ||||
| E.coli |
16 |
16 |
16 |
4 |
| P. aeruginosa |
64 |
64 |
64 |
8 |
| S. typhimurium |
16 |
16 |
16 |
4 |
MIC: Minimum inhibitory concentration
Kanamycin: antibiotic
Conclusion
Two new natural biphenylpropanoid analogous 1 and 2 (named biphenylsalsonoids A and B) were isolated from the roots of Salsolavillosa. Their structures were elucidated by spectroscopic methods including 1D, 2D-NMR experiments. Compounds 1 and 2 showed a moderate activityof radical-scavenging towards DPPH and ABTS. The ABTSscavenging activity of compounds was similar to the DPPH free radical scavenging. Their antibacterial activity was evaluated by the MIC method against Staphylococcus aureus, S. epidermidis, Micrococcus luteus, Escherichia coli, Salmonella typhimurium and Pseudomonas aeruginosa. The two compounds have shown the same activity towards the tested bacteria, except M. luteus which exhibited more sensitivity against compound 2.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at Northern Borders University for its funding of this research through the research project No. SCI-2016-F-5705.
References
- Botschantzer, V.P.A. synopsis of Salsola(Chenopodiaceae) from South and South-West Africa. Kew Bulletin.1974, 29,597-604.
CrossRef - Vladimir, I. P.; Elena, V.V.; Alexander, N.K.; Maurice. S.B; Eric, G; Vincent, R. F.; Clanton, C. B.; Gerald, E. E. Occurrence of C3 and C4 photosynthesis in cotyledons and leaves of Salsolaspecies (Chenopodiaceae). Photosynthesis Research. 2000,63, 69-84.
CrossRef - Mabberley, D. J. The plant book. A portable dictionary of the vascular plants (2nd Edition), Cambridge university press, London. 1997,149, 260
- Al-Saleh, F.S.; Ali, H.; Mirza, M. Chemical constituents of some medicinal plants growing in Bahrain. Fitoterapia.1993,64, 251-256.
- Borkowski, B.;Wrocinski, T.Porownaniedzialaniahipotensyjunegosalsoliny I salsolidynyorazprotoweralyren. Actal. Polon. Pharm. 1959,16, 197-203.
- Tundis, R.;Menichini, F.Confort, F.;Loizzo, M.R.;Bonesi, M.;Statti, G.;Menichini, F.A. potential role of alkaloid extracts from Salsola species (Chenopodiaceae) in the treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2009, 24, 818-824.
CrossRef - Ahmad, Z.; Mehmood, S.; Fatima, I.; Malik, A.; Ifzal, R.; Afza, N.; Iqbal, L.; Latif, M.; Nizami, T.A. Structural determination of salsolins A and B, new antioxidant polyoxygenatedtriterpenes fromSalsolabaryosma by 1D and 2D NMR. Spectroscopy.Magn.Reson. Chem. 2008, 46(1), 94-98
CrossRef - Syrchina, A.I.; Vereshchagin, A.L.;Larin, M.F.; Semenov, A. Flavonoids of Salsolacollina. Compounds Chemistry of Natural.1989,25(5), 619-620.
CrossRef - Tundis, R.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Inhibitory effects on the digestive enzyme alpha-amylase of three Salsolaspecies (Chenopodiaceae).in vitroPharmazie. 2007, 62, 473–475. 271
- Arafa, I.; Hamed, M.M.; Mohamed G.S.; Usama A.; Moatz M.T.; Angela, P.; Sonia, P.Triterpenesaponins from Salsola imbricate. PhytochemistryLetters.2011, 4, 353–356.
- Ahlem, B.; Atef Ch.; Hatem G.; M’hamed, A.H.; Hichem, B.J.New antioxidant bibenzyl derivative and isoflavonoid from the Tunisia SalsolatetrandraFolsk.Natural product research.2012, 26(3), 235-242.
CrossRef - Oueslati, M.H.; Al-Ghamdi, F.A.; Noubigh, A.Two new bioactive salsolanol and biphenylsalsinol from the aerial parts of SalsolavillosaDelile.exSchul. (Chenopodiaceae) growing in Saudi Arabia.Asian Pac. J. Trop. Biomed. 2015,5(8), 624–628.
CrossRef - Oueslati, M.H.; Ben Jannet, H.; Abreu, P.; Mighri. Z. J. Soc. Alger. Chim. 2004, 14, 181-187.
- Oueslati, M.H; Hichem, B.; Zine, M.; Chriaa, J.; Pedro, M. PhytochemicalConstituentsfromSalsolatetrandra. J. Nat. Prod. 2006, 69, 1366-1369.
CrossRef - Osman, S.M.; El Kashak, W.A.; Wink, M.; El Raey, M.A. New isorhamnetin derivatives from SalsolaimbricataForssk.leaves with distinct anti-inflammatory activity.Pharmacognosy magazine.2016, 12, 47-51.
CrossRef - Iwaki, K.; Koya-Miyata; Kohno, K; Ushio, S.; Fukuda, S. Antimicrobial activity of PolygonumtinctoriumLour. extract against oral pathogenic bacteria. Nat. Med. 2006, 53, 72-79.
- Tripathi, V.D.;Agarwal,S.K.;Rastogi, R.P. An antibacterial biphenyl derivative and other constituents ofAtylosiatrinervia. Phytochemistry.1978, 17, 2001-2003.
- Indu, M.N.; Hatha, A.A.M.; Abirosh, C.; Harsha, U.; Vivekanandan, G. Antimicrobial activity of some of the south-Indian spices against serotypes of Escherichia coli, Salmonella, Listeriamonocytogenesand Aeromonashydrophila. Braz. J. Microbiol.,2006, 37, 153-158.
CrossRef - Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem.2005, 90, 333–340.
CrossRef - Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radical Bio. Med. 1999, 26, 1231–1237.
CrossRef - Wen-Zhe, H.; Chao-Feng, Z.; Mian, Z.; Zheng-Tao, W.A New Biphenylpropanoid from Alpiniakatsumadai.Journal of the Chinese Chemical Society.2007, 54, 1553-1556.
CrossRef - Ribeiro, P.R.; Ferraz, C.G.; Guedes, M.L.S.; Martins, D.; Cruz, F.G. A new biphenyl and antimicrobial activity of extracts and compounds from Clusiaburlemarxii. Fitoterapia.2011, 82, 1237-1240
CrossRef - Jun, L.; Yan, H.; Xin-Lan, G.; Jian, L.; Sheng-Ping, D.; Qiang, W.; Yan-Jun, N. Z.; Xiao-Jia, S.; Rui-Yun, Y. Anti-hepatitis B virus constituents from the stem bark of Streblusasper. Phytochemistry.2012, 82, 100-109.
CrossRef
- Siridechakorn, I.; Maneerat, W.; Sripisut, T.; Ritthiwigrom, T.; Cheenpracha, S.; Lapho, O. S. Biphenyl and xanthone derivatives from the twigs of a Garcinia sp. (Clusiaceae). PhytochemistyLetters.2014, 8, 77-80.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









