Production of Macrocyclic Polyether Benzo-15-Crown-5 and its Functional Derivatives
Valentina N. Glushko, Natalya Yu. Sadovskaya, Vadim I. Kozhuhov, Lidiya I. Blokhina, Irina A. Antropova, Ekaterina S. Petina, Vasiliy М. Retiviov, and Ekaterina Yu. Melnikova
The Federal State Unitary Enterprise «Institute of Chemical Reagents and High Purity Chemical Substances of National Research Centre «Kurchatov Institute», Moscow, Bogorodsky val str.3.
Corresponding Author E-mail: tetrazoli@yandex.ru
DOI : http://dx.doi.org/10.13005/ojc/330413
Study of interaction between catechol and tetraethyleneglycol dichloride in the n-butanol media, resulting with benzo-15-crown-5 production. Production of nitro- and amino-derivatives of benzo-15-crown-5. Determination of their thermogravimetric characteristics.
KEYWORDS:benzo-15-crown-5; catechol, tetraethyleneglycol dichloride; crown ether; gas liquid chromatography; chromate-mass spectrometry
Download this article as:| Copy the following to cite this article: Glushko V. N, Sadovskaya N. Y, Kozhuhov V. I, Blokhina L. I, Antropova I. A, Petina E. S, Retiviov V. M, Melnikova E. Y. Production of Macrocyclic Polyether Benzo-15-Crown-5 and its Functional Derivatives. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Glushko V. N, Sadovskaya N. Y, Kozhuhov V. I, Blokhina L. I, Antropova I. A, Petina E. S, Retiviov V. M, Melnikova E. Y. Production of Macrocyclic Polyether Benzo-15-Crown-5 and its Functional Derivatives. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=34436 |
Introduction
The results of studying crown ethers, which were discovered in 1962, allowed to reveal their properties ensuring their successful use for solving issues in various fields of science, medical practice and modern manufacturing. A unique property of crown ethers is their high selectivity in terms of forming host-guest complexes with metal ions and neutral organic molecules. Such interaction is specific and determined by both electronic and topological factors, which opens broad prospects for using crown ethers as highly selective splitters, carriers, catalyst and the like [1-6 ]. These properties has already helped crown ethers secure their place in medicine [7], botany [8], engineering and technology [9].
It is possible to synthesize crown-ether-based adsorbing agents, and their adsorbing ability could be further improved due to the crown-ether fragments chemically attached to them. Non-soluble polymers with crown units in their backbone or side chains turn out to be preferable over their monomer analogues, since they are easier to process, regenerate and reuse
For example, adding ether groups to chloromethylated styrene/divinylbenzene copolymer enabled to produce electrodialysis membranes capable of adsorbing alkali metal chlorides and releasing them after slight temperature increase.
Amino- and diamino-crown ethers and nitrogen-containing heterocyclic compounds suitable for further functional improvement are of particular interest, since there compounds can immobilize into various organic and non-organic carriers and also act as an initial material for forming new nitrogen-containing structures.
Amino-substituted crown compounds can also enter acylation, phosphorylation, addition, condensation, alkylation and arylation reactions, form isocyanates under phosgene effect and can be immobilized on carrier surface [3].
Despite growing interest in these compounds over recent years, the current number of papers on the synthesis of macrocyclic polyethers is extremely small [10-15]. For example, benzo-15-crown-5 is produced using Williamson synthesis reaction, featured by interaction between catechol (orto-dihydroxybenzene) and tetraethyleneglycol dichloride (1,10-dichloro-3,6,9-trioxadecane) over sodium hydroxide in the n-butanol media (30-hour inert-atmosphere boiling). The yield of benzo-15-crown-5 produced by this method is 62%, with the basic substance’s mass fraction of 95%.
Nitro-derivative B15C6 is produced by applying 76% HNO3 in a glacial acetic acid. This method was used for producing 4′-nitro-B18C6, 4′- nitro-B15C5 and 4′,5′-dinitro-B15C5 and nitro-derivatives B18C6 respectively [16]. Mononitro-derivatives B18C6 and B15C5 too were synthesized using 58% HNO3 in acetonitrile [17].
The relevant literature contains only a few main methods of producing amino-derivatives of benzo- and dibenzo-crown ethers. In one of their latest papers [18] the authors reduced dinitro-derivatives of crown ethers by hydrazine hydrate over palladium on carbon in the diethylene glycol dimethyl ether media.
In case of paper [19], the reduction was carried out over palladium on carbon in dimethylformamide, with its duration and pressure of 1.75 hours and 1551.49 torr respectively.
Feigenbaum and Michel [20] produced cis- and trans-isomers of diaminodibenzo-18-crown-6 by hydrogenating corresponding individual dinitro-isomers in the form of suspension over Raney nickel in dimethylformamide, using pressurized hydrogen. Figure 1 shows 4-aminobenzo-15-crown-5 synthesis scheme.
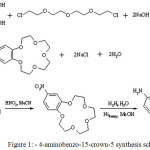 |
Figure 1: 4-aminobenzo-15-crown-5 synthesis scheme Click here to View figure |
A series of experiments was conducted to determine the optimal conditions for performing benzo-15-crown-5 production reactions.
For the purpose of ensuring selective behavior of cyclization reaction, which results with cyclic compounds, and inhibiting side reactions that lead to formation of linear polymers, a high dilution method is used for synthesizing crown ethers, since the formation of macrocyclic product through intramolecular reaction in diluted solutions is more likely, so this process will be faster than polymer formation, which requires collisions between two reagents (intermolecular reaction) [21-22].
Since sodium hydroxide is used as a template agent in production reaction, a method of adding tetraethyleneglycol dichloride (TEGDC) plays an important role too, due to its possible hydrolysis in the alkali media. Therefore, three methods of adding this reagent to the reaction (dropwise addition; adding 1/3 of tetraethyleneglycol dichloride amount every ten minutes; and adding the whole amount of tetraethyleneglycol dichloride at once) were studied. A thermodynamic condition of the reaction mixture during dichloride addition varied too.
The following reagents were using for synthesizing benzo-15-crown-5: catechol, 99.6% (manufacturer: Merck); tetraethyleneglycol dichloride, 98.5%; n-butanol, 99.5%; sodium hydroxide, 99.5%; hydrochloric acid, 38%; and pure-grade hexane.
Benzo-15-Crown-5 Synthesis (Synthesis 1: Dropwise Addition of Tetraethyleneglycol Dichloride)
General Procedure
Into a 2-liter flask equipped with a mechanical stirrer, thermometer, water condenser and dropping funnel was placed 88 g (0.8 mole) catechol and 1200 ml n-butanol. After dissolution of the catechol by stirring, aqueous NaOH solution (67.2 g NaOH to 80 ml H2O) was added. Mixing duration was 30 to 40 minutes. Then 156.6 ml (0.8 mole) tetraethyleneglycol dichloride was dripped through dropping funnel at 17 ml/min rate. The mixture was refluxed for 7 hours (107 ˚С). After that, the filtrate was acidified with concentrated hydrochloric acid, filtered off and flushed with water until neutral reaction was reached. Upper organic layer was put into round-bottom flask, treated with activated carbon and filtered off. Then the filtrate was boiled out using rotary evaporator, accompanied by complete solvent distillation. The residue was extracted with hexane, followed by producing white transparent crystals of benzo-15-crown-5.
Infrared spectrum (KBr, ν, cm-1): 468 (weak), 514 (weak), 538 (weak), 602 (weak), 740 (strong) (С-Н аром), 779 (weak), 851 (medium), 906 (weak), 938 (strong) (aromatic С-Н), 980 (weak), 1042 (medium), 1051 (strong), 1076 (medium), 1094 (medium), 1121 (strong) (С-О-С), 1130 (strong) (С-О-С), 1226 (strong) (aromatic С-Н), 1261 (strong) (aromatic С-Н), 1269 (medium), 1335 (medium), 1345 (medium), 1362 (weak), 1412 (weak), 1455 (medium), 1509 (strong) (СН2), 1593 (medium), 2863 (strong) (С-Н), 2920 (strong) (С-Н), 2940 (strong) (С-Н), 3009 (weak) (С-Н aromatic), 3036 (weak) (С-Н aromatic), 3060 (weak) (С-Н aromatic)
1Н Nuclear magnetic resonance (DMSO-d6, 300 MHz): 3.62 (broad singlet, 8Н, СН2+СН2+СН2+СН2), 3.73-3.81 (multiplet, 4Н, СН2+СН2), 4.00-4.09 (multiplet, 4Н, СН2+СН2), 6.85-6.99 (multiplet, 4Н, aromatic СН).
13С Nuclear magnetic resonance (DMSO-d6, 75 MHz): 68.42, 68.87, 69.79, 70.42, 113.92, 121.04, 148.63.
Benzo-15-Crown-5 Synthesis (Synthesis 2: (Adding Tetraethyleneglycol Dichloride in three Equal Parts)
The process is similar to Synthesis 1 (dropwise addition). The only difference is that tetraethyleneglycol dichloride was added to the reaction mixture by 52.2 ml each 10 minutes.
Benzo-15-Crown-5 Synthesis (Synthesis 3: Adding the Whole Amount of Tetraethylenglycol Dichloride)
The process is similar to Synthesis 1. The only difference is that 156.6 ml tetraethyleneglycol dichloride was added to the reaction mixture at once.
Benzo-15-Crown-5 Synthesis (Synthesis 4: Dropwise Addition of Tetraethyleneglycol Dichloride to Boiling Reaction Mixture)
The process is similar to Synthesis 1. The only difference is that reaction mixture was heated to the boiling point prior to dichloride addition.
Benzo-15-Crown-5 Synthesis (Synthesis 5: Adding Tetraethyleneglycol Dichloride to Boiling Reaction Mixture in three Equal Parts)
The process is similar to Synthesis 2. The only difference is that reaction mixture was heated to the boiling point prior to dichloride addition.
Benzo-15-Crown-5 Synthesis (Synthesis 3: Adding the Whole Amount of Tetraethyleneglycol Dichloride to Boiling Reaction Mixture)
The process is similar to Synthesis 2. The only difference is that 156.6 ml tetraethyleneglycol dichloride was added to the reaction mixture, which, in turn, was preheated to the boiling point.
Reaction flow monitoring was carried out using GLC method. Molar concentration values of target product within reaction mixture were determined using gas liquid chromatography (Table 1):
For the purpose of studying the kinetics of benzo-15-crown-5 production process, reaction mass sampling was carried out at fixed time intervals, using alcohol solution of hydrochloric acid as a stop reagent (pH=3-4).
Table 1: Target product percentage of the reaction mixture depending on reaction conditions
|
Method of adding tetraethyleneglycol dichloride (TEGDC) to reaction mass |
B15C5 content of reaction mass, % |
|
|
TEGDC addition to boiling reaction mass |
TEGDC addition at room temperature |
|
| Dropwise TEGDC | 82.097 | 72.902 |
| 1/3 of TEGDC amount every ten minutes | 80.192 | 65.482 |
| Full amount of TEGDC at once | 78.431 | 58.934 |
Quantitative sample analysis was carried out using gas liquid chromatography (GLC), which, in turn, was performed using gas chromatograph «Chromatec Crystal 5000.2», equipped with flame ionization detector and 30-m long fused silica column ВР-5, with its inner diameter and fixed-phase film thickness of 0.32 mm and 0.5µm respectively. Carrier has: helium (rate: 2.8 ml/min, split ratio: 1:25, column temperature range: 150-300ºС, temperature setting: 10ºС/min. Concentration values of sample components were calculated using percentage normalization per area.
4-Nitrobenzo-15-Crown-5 Production (NB15C5)
Benzo-15-crown-5 nitration was carried out by nitric acid, 58%, over boiling reaction mass in the acetonitrile media, using relevant method [17].
Nitric acid of various concentrations can be used in nitration reaction. In some cases diluted nitric acid, which is poor nitration agent and quite strong oxidizing agent, due to the constant presence of nitrous acid that hinders nitration, is used as well. Another drawback of diluted nitric acid, which limits its use, is its ability to express nitrating effect only at higher temperatures, which are featured by prevailing side oxidation reaction. Concentrated nitric acid shows nitrating effect at lower temperatures and less likely to cause undesirable oxidation, which is why we carried out a nitration of resulted benzo-15-crown-5 with nitric acid, 56%, in the acetonitrile media. The yield of target product was 99.5%, while the melting point of resulted compound was 94-95ºС.
Infrared spectrum (KBr, ν, cm-1): 497 (weak), 538 (weak), 618 (weak), 654 (medium) (aromatic С-Н), 723 (weak), 745 (medium) (NO2), 787 (weak), 806 (medium), 868 (medium) (N-O), 913 (weak), 934 (medium), 980 (medium), 1010(weak), 1047 (medium), 1093 (strong) (С-N), 1138 (strong) (С-О-С), 1240 (strong) (NO2), 1276 (strong) (NO2), 1335 (strong) (NO2), 1417 (strong), 1428 (strong), 1448 (medium) (N=O), 1517 (strong) (СН2 + NO2), 1587 (medium) (aromatic С-С), 2868 (medium) (С-Н), 2904 (medium) (С-Н), 2929 (medium) (С-Н), 3083 (medium) (aromatic С-Н).
1Н Nuclear magnetic resonance (DMSO-d6, 300 MHz): 3.62 (broad singlet, 8Н, СН2+СН2+СН2+СН2), 3.74-3.85 (multiplet, 4Н, СН2+СН2), 4.13-4.24 (multiplet, 4Н, СН2+СН2), 7.15 (doublet, 1Н, J=8.9, СН), 7.73 (doublet, 1Н, J=2.7, СН), 7.89 (double doublet, 1Н, J=8.9, J=2.7, СН).
13С Nuclear magnetic resonance (DMSO-d6, 75 MHz): 67.77, 68.84, 69.27, 69.71, 70.09, 70.16, 70.30, 100.96, 105.53, 117.49, 139.35, 143.95, 149.92.
4-Aminobenzo-15-Crown-5(AB15C5) Production
For the purpose of reducing 4-nitrobenzo-15-crown-5 we chose the method of reduction by hydrazine hydrate over skeleton Raney nickel catalyst in the alcohol media as the most convenient and effective one. Contrary to other methods, a reduction by hydrazine hydrate over nickel catalyst takes place at normal pressure and without autoclave.
5 g Raney nickel was added to 34 g (0.108 mole) 4-nitrobenzo-15-crown-5 within 200-ml ethyl alcohol and heated to 500 ºС, followed by dripping 34 ml hydrazine hydrate. In one hour’s time it was filtered off the catalyst, with solution boiled out and residue recrystallized from 30-ml ethyl alcohol. The yield of AB15C5 was 70%, with its melting point of 79.5-80.5 ºС.
Infrared spectrum (KBr, ν, cm-1): 497 (weak), 581 (weak), 631 (weak), 712 (weak), 766 (weak), 793 (weak) (NH2), 847 (medium) (NH2), 917 (weak), 940 (medium), 987 (medium), 1060 (medium) (С-О-С), 1079 (medium), 1127 (strong) (С-О-С), 1181 (medium), 1217 (medium) (С-N), 1251 (medium), 1294 (medium), 1332 (weak), 1355 (weak), 1452 (medium), 1511 (strong) (СН2), 1589 (weak) (С-С аром), 1610 (medium) (NH2), 2878 (medium) (С-Н), 2921 (medium) (С-Н), 2957 (medium) (С-Н), 3056 (aromatic С-Н), 3346 (strong) (NH2), 3396 (strong) (NH2).
1Н Nuclear magnetic resonance (DMSO-d6, 300 MHz): 3.60 (broad singlet, 8Н, СН2+СН2+СН2+СН2), 3.68-3.77 (multiplet, 4Н, СН2+СН2), 3.85-3.97 (multiplet, 4Н, СН2+СН2), 4.67 (broad singlet, 2Н, NH2), 6.05 (double doublet, 1Н, J=8.4, J=2.5, СН), 6.22 (doublet, 1Н, J=2.5, СН), 6.63 (doublet, 1Н, J=8.4, СН).
13С Nuclear magnetic resonance (DMSO-d6, 75 MHz): 67.77, 68.84, 69.27, 69.71, 70.09, 70.16, 70.30, 100.96, 105.53, 117.49, 139.35, 143.95, 149.92.
Results and Discussion
Reaction products were identified using gas chromatograph «Chromatec Crystal 500.2», equipped with mass spectrometry detector ThermoISQ and 15 m long fused silica column TR-5MS, with its inner diameter and fixed-phase film thickness of 0.25 mm and 0.25µm respectively. Carrier has: helium (rate: 2.8 ml/min, split ratio: 1:25, column temperature range: 150-280 ºС, temperature setting: 20 ºС/min. Mass spectrums of an electronic impact were determined at the 70-eV energy of ionizing electrons and 280°С temperature of ion source. Reaction products were identified mainly by comparing registered spectrum with that of NISTUSA (2008) and also by molecular ion based on the correlation between mass, spectrum and structure.
Reaction products were identified using chromate-mass spectrometry
It was found out that there were two impurities, identified as compounds A and B, which formed alongside with benzo-15-crown-5. The formation of impurity A was the result of tetraethyleneglycol dichloride hydrolysis, while impurity B was 2-(2-(2-(2-(2-chlorethoxy)ethoxy)ethoxy)ethoxy)phenol. Structural formulas of these side products are shown in Figures 2 and 3 below.
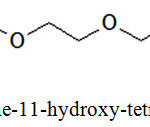 |
Figure 2: 1-chlorine-11-hydroxy-tetraethyleneglycol, m/z 212.5 Click here to View figure |
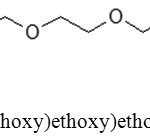 |
Figure 3: 2-(2-(2-(2-(2-chlorethoxy)ethoxy)ethoxy)ethoxy)phenol, m/z 304.11 Click here to View figure |
In case of Synthesis 1, apart from target product (i.e. benzo-15-crown-5 (80.09%)), intermediate and side products, the reaction mixture also contained initial catechol and tetraethyleneglycol dichloride (4.149% and 8.106% respectively) at 6.5 hours’ time after the reaction had started. As synthesis duration increased to 8.5 hours, the content of target product reached 82.097%, with its further formation taking place at extremely low rate.
In case of adding tetraethyleneglycol dichloride in three equal parts every 10 minutes, the concentration of target product under the same conditions was 80.192%. In case of adding the whole amount of tetraethyleneglycol dichloride at once, the concentration of target product was 78.431%.
The curve of dependence between reaction mixture and synthesis duration 1 is shown in Figure 4.
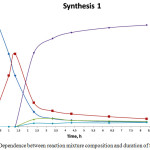 |
Figure 4: Dependence between reaction mixture composition and duration of Synthesis 1 Click here to View figure |
Thus, benzo-15-crown-5 forms only after reaction mixture has been heated to its boiling point (107ºС), which takes 1.3 hours. Then, the temperature of reaction mixture decreases to 102ºС, followed by formation of two side products, A и B, in comparatively small quantities (4.211% and 3.554% respectively).
Benzo-15-crown-5 is extracted from boiling reaction mass by hexane. The mass fraction of resulted product’s basic substance was 98%, with its melting point of 79.5-80.5 ºС.
Given the kinetics of Sythesis 1, we assumed that TEGDC addition to boiling reaction mixture should accelerate the formation of crown ether and increase its yield. However, this assumption proved to be wrong, since dropwise addition of tetraethyleneglycol dichloride to boiling reaction mixture resulted in target product’s concentration after 7 hour boiling of 72.902%, which was 9.195% lower than that of similar synthesis with TEGDC addition not accompanied by boiling. In case of adding TEGDC in three parts, the concentration of target product was 65.482%. In case of adding the whole amount of TEGDC at once, the concentration of target product was 58.934%.
Thus, the best conditions for benzo-15-crown-5 synthesis among the above-mentioned ones are adding tetraethyleneglycol dichloride to unboiled reaction mass.
The chosen methods for the preparation of nitro- and amino-derivatives of benzo-15-crown-5 make it possible to obtain the above-mentioned functional crown ethers with high yields and high quality.
Differential thermal analysis was used for determining melting points and analyzing thermal decomposition process of B15C5, NB15C5 and AB15C5. TGA (thermo-gravimetric analysis) and DSC (differential scanning calorimetry) methods, as well as combined simultaneous thermal analyzer STD Q600 (manufacturer: TA Instruments (USA)) were used in these study. Measurements were carried out in air (flow rate: 100 ml/min) inside alundum crucibles, with the heating rate of 10ºС/min and the temperature ranging from room temperature to 300ºС.
Thermal decomposition of crown-ethers is a complicated and staged process which comprises 2 stages: compound melting, which takes place at temperatures of up to 100ºС, compound decomposition, which begins within temperature range of 150-175ºС.
Figure 5 shows the thermograph of sample B15C5. An endothermic peak at a temperature of about 80ºС, not accompanied by massive changes, means the melting process. Decomposition begins at 150ºС, with 14% mass loss at the first stage. At 217ºС decomposition process accelerates and lasts until the whole mass is lost. There is a narrow exothermic peak at 274 ºС, which point to possible ignition of the substance.
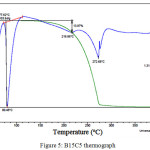 |
Figure 5: B15C5 thermograph |
Figure 6 shows the thermograph of sample NB15C5. An endothermic peak at a temperature of about 97 ºС, not accompanied by massive changes, means the melting process. An exothermic peak at a temperature of about 314 ºС, accompanied by complete loss of mass, points to sample combustion during decomposition process.
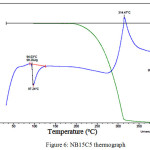 |
Figure 6: NB15C5 thermograph Click here to View figure |
Figure 7 shows the thermograph of sample AB15C5. An endothermic peak at a temperature of about 97ºС, accompanied by 0.5% massive loss, points to a water loss process taking place alongside melting. The decomposition of this sample is complicated and comprises two stages. At the first stage, there is a rapid mass loss of 58% within the temperature range of 170-297ºС. Then the process slows down, with 77.7-% loss of sample mass at the temperature of 400ºС.
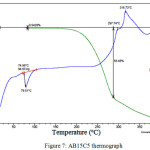 |
Figure 7: AB15C5 thermograph
|
Differential thermal analysis was used for determining melting points and analyzing thermal decomposition process of B15C5, NB15C5 and AB15C5. TGA (thermo-gravimetric analysis) and DSC (differential scanning calorimetry) methods, as well as combined simultaneous thermal analyzer STD Q600 (manufacturer: TA Instruments (USA)) were used in these study. Measurements were carried out in air (flow rate: 100 ml/min) inside alundum crucibles, with the heating rate of 10ºС/min and the temperature ranging from room temperature to 300ºС.
As it was shown, the melting point of B15C5 was 80.48ºС. Sample mass is reduced by 0.04 % in the course melting process. In case of NB15C5, melting point and mass loss were 97.24ºС and 0.44 % respectively. In case of AB15C5, melting point and mass loss were 79.51ºС and 0.53%.
Conclusion
The study has shown that adding tetraethyleneglycol dichloride to the reaction mixture at the rate of 17 ml/min results in benzo-15-crown-5 content reaching 82.097% of reaction mass. At the same time, we have proved that 7 hours are an optimal dwell time at the boiling point, which allows to reduce reaction duration by 23 hours, comparing with conventional method. It has also been found that it is possible to carry out the reduction of nitrobenzo-15-crown-5 by using hydrazine hydrate over Raney nickel at normal atmospheric pressure. Thermogravimetric properties of resulted products have been determined.
Acknowledgments
Applied researches are carried out with state financial support represented by the Ministry of Education of Russia under the Agreement on granting subsidies №14.625.21.0034 of October 27, 2015. (Unique identifier of Applied Scientific Researche (project) RFMEFI62515X0034).
References
- Steed, J.W.; Atwood, J.L. Supramol. Chem. 2007, 1, 121-126
- Hiraoka, M. Crown compounds. Properties and application.‑ Moscow, Mir, 1986
- Yatsimirsky, K.B.; Kolchinsky, A.G.; Pavlishuk, V.V.; Talanova, G.G. Synthesis of macrocyclic compounds.‑ Kyiv, Naukova Dumka, 1987
- Marijeta, K.; Ljerka, T.; Frkanec, L. Chem. Med. Chem. 2008, 3, 1478-1492.
CrossRef - Pliego, J. R.; Riveros, J.M. J. Mol. Catal. A: Chem. 2012, 15(4), 489-494.
CrossRef - Rounaghi, G.; Eshghi, H. Arab. J. Chem. 2012, 17(7), 378-379
- Yu, H.-R.; Ju, X.-J.; Xie, R.; Wang, W.; Zhang, B.; Chu, L.-Y. Anal. Chem. 2013. 85(13), 6477-6484.
CrossRef - Bogatsky, A.; Lukyanchenko, N. Fiziologiya Rasteniy. 1984, 6, 1015-1020
- Amiran, M.C. Chemical cleaning solution for gas turbine blades. Patent US 6310022 B1. 2001
- Kotlyar, S.A.; Gorodnyuk, V.P.; Grigorash, R.Ya.; Chuprin, G.N. Russ. J. Gen. Chem. 1998, 68(7), 1189-1192
- Piatek, P.; Litwin, A. Tetrahedron. 2009, 65, 2285-2289.
CrossRef - Shen, Y.C.; Shih J.S. J. Chin. Chem. Soc. 2008, 55, 578-586.
CrossRef - Sousa, C.; Gameiro, P.; Freire, C.; Castro, B. Polyhedron. 2004, 23, 1401-1408.
CrossRef - Titova, Yu.A.; Fedorova, O.V.; Ovchinnikova, I.G.; Maksimovskikh, A.I.; Uimin, M.A.; Rusinov, G.L.; Charushin, V.N. Macroheterocycles. 2014, 7(1), 23-27.
CrossRef - Lowicki, D.; Huczynski, A.; Stefanska, J.; Brzezinski, B. Tetrahedron. 2011, 67, 1468-1478.
CrossRef - Ungaro, R.; Haj B. El.; Smid, J. J. Am. Chem. Soc. 1976, 98, 5198.
CrossRef - Ivanov, O.V.; Markevitch, I.S.; Blokhina, L.I.; Nikolaenko, S.P.; Filatova, M.P.; Vasilchenko, G.V. Preparation of mononitrobenzo-crown ethers. Patent USSR 1544774 A1. 1990
- Martyanov, T.P.; Ushakov, E.N.; Savelyev, V.A.; Klimenko, L.S. Russ. Chem. Bull., Intern. Ed. 2012, 61(12), 2261-2273
- Deetz, M.J.; Shang, M.; Smith, B.D. J. Am. Chem. Soc. 2000, 122, 6201-6207.
CrossRef - Feigenbaum, W.M.; Michel, R.H. J. Polym. Scien. P. A-1: Polym. Chem. 1971, 9(3), 817-820
- Wang, D.; Xing, J.; Peng, J.; Wu, C. J. Chromatography A. 2003, 1005, 1-12.
CrossRef - Rossa, L.; Vögtle Fr. Chem. Med. Chem. 2014, 8(1), 3-5

This work is licensed under a Creative Commons Attribution 4.0 International License.









