NMR, ESR, NQR and IR Studies of Paramagnetic Macro Cyclic Complexes of 1st Transition Series Metal Lons Exhibiting MLCT Phenomenon: A DFT Application: Part: II. Bis (1, 10-Phenanthroline) Complexes
M. L. Sehgal1 and Irshad Ahmad2
1Fmr. Head Chemistry, D.A.V. College, Jalandhar-144008, India.
2Department of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, 202002, Uttar Pradesh, India.
Corresponding Author E-mail: irshadahmad.bio@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330416
D F T implemented in ADF.2012.01 was used to study the structures of 8 macro cyclic paramagnetic four coordinate D2d complexes:[Phen2M]2+ {M= Mn (II), Fe(II), Co(II),Ni(II)(II),Cu(II)};[Phen2M]3+{M=Ti(III),V(III),Cr(III)}(Phen=1,10-phenanthroline) by applying four spectral techniques. After pre-optimization of complexes, the software was run by using Single Point, LDA or GGA, Default, Relativity, Spin Orbit, ZORA , Unrestricted, None, Collinear ,Nosym using TZP or TZ2P Basis sets in ESR/EPR/EFG/ZFS Program to obtain ESR parameters: g11, g22, g33, giso, a11, a22, a33, A ten. More ESR parameters (gn. A ten, ZFS) and NQR parameters {h, q11, q22, q33, NQCC} were obtained on replacing Spin Orbit by scalar command in a new ADF Input. The “NMR Program” with Single Point, LDA, Default, None, Collinear, Nosym using DZ or TPZ Basis sets leaving Unrestricted command blank gave the Shielding Constants (σM, σ 13C,σ 17O), Chemical Shifts (δ M, δ13C, δ17O),2 diamagnetic ,4 paramagnetic and 4 spin orbit contributing terms in the σ values of the constituents. The software was, then, run with Frequencies to obtain IR frequencies of normal modes of each of 129 fundamental vibration bands of the complexes. Importance of the study would lie in the fact that: (i) 5 parameter: σ, δ,h, gn .A ten, NQCC of 14N; 4 parameters: σ, δ, g n. A ten, h of 13C and 3 parameters: σ, δ, g. A ten of 1H corroborated to infer that in all these complexes, the 24 C were of 6 types; the16 H of 4 types and all the 4 N were spatially of the same type(ii) it confirmed MLCT phenomenon by NMR(iii) calculated another parameter of each of NMR (H^) and ESR {ΔEhf} and two of NQR {h,Laplace equation} (iv) classified129 bands into vibration symmetries and IR activities and (iv) gave thermal parameters of the complexes.
KEYWORDS:Chemical Shift; Total NMR Shielding Tensor; Effective Spin Hamiltonian; Nuclear Quadrupole Coupling Constant; Asymmetric Coefficient
Download this article as:| Copy the following to cite this article: Sehgal M. L, Ahmad I. NMR, ESR, NQR and IR Studies of Paramagnetic Macro Cyclic Complexes of 1st Transition Series Metal Lons Exhibiting MLCT Phenomenon: A DFT Application: Part: II. Bis (1, 10-Phenanthroline) Complexes. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Sehgal M. L, Ahmad I. NMR, ESR, NQR and IR Studies of Paramagnetic Macro Cyclic Complexes of 1st Transition Series Metal Lons Exhibiting MLCT Phenomenon: A DFT Application: Part: II. Bis (1, 10-Phenanthroline) Complexes. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35627 |
Introduction
Aromatic heterocyclic compounds such as 1, 10-phenanthroline (Phen) and 2, 2-bipyridine (Bipy) form an important class of compounds in which the π and n electrons can form charge-transfer complexes [1, 2] where the electronic charge of their complexes having filled π- molecular orbitals of energy comparable to the metal orbitals get transferred into the empty molecular orbitals with energy nearly equal to the ligand orbitals. They absorb in the visible region to exhibit intense color which is termed as Metal to Ligand Charge Transfer (MLCT) phenomenon.
Unlike 2,2/-bipyridine, the1,10-phenanthroline does not have the same conformational flexibility and it would bind metal ions more strongly [3,4].This enantioselective interaction of 1, 10-phenanthroline complexes of iron (II), Co (III), Zn (II)is used as a structural probe and mediators of DNA cleavage reactions [5-7]. Homoleptic bis(1,10-phenanthroline) transition metal ions complexes [Phen2M] 2+, 3+ were studied comparatively to a lesser extent both experimentally and by Density Functional Theory(DFT). Single crystal structures of bis [chloro bis(1,10-phenanthroline-N,N′)(thiourea -S) nickel(II)] chloride nitrate was studied by L. Suescun and his coworkers[8]. Structure and magnetic properties of di-μ-hydroxo-bis [bis(1,10-phenanthroline)chromium(III)] iodide tetrahydrate were studied by Raymond Scaringe and Phritu Singh[9]. O. Yesilel, H. Olmez studied spectrothermal properties of 1, 10-phenanthroline complexes along with X-rays single crystal structure and photo luminance properties of Co (II), NI (II), Cu (II) and Cd (II) orotates with UV-Vis and FTIR [10].V. Chis et al [11] studied Spin-state cross-over in Fe (Phen2(NCS)2 by DFT. Ozel Aysen E. and coworkers [12]studied vibration spectra of copper(II)2,2’-bipyridine and 1,10-phenanthroline complexes by applying DFT[12]. DFT IR, UV and Mossbauer of 2, 2’-bipyridine and 1, 10-phenanthroline complexes of iron (II) was studied by Morigakii, Milton K. et al [13] with the help of DFT.H.Z. Chiniforoshan et al [14] studied the structures of complexes of Co (III) and Zn (II) with 2,2’-bipyridine and 1,10-phenanthroline by applying DFT. Sumit Sanotra et al applied DFT to study X-ray crystal structure and photo luminance properties of [Cd (NO3)2 (phen)2][15].Euan K. Bre chin and coworkers[16] studied UV,ESR and magnetic properties of Cr(III), Fe(II).N.S. Panina and coworkers[17] studied stabilities of high and low spin isomers and X-ray structures of Ni(II) complexes. Zn Bz Li and Xiao yuan studied Raman spectra of bis- and tris (1, 10-phenanthroline) manganese (II) complexes [18].
Need for the Study
(a) A limited experimental research had been carried out in the calculation of NMR, ESR and NQR parameters of these 8 macro cyclic paramagnetic 1,10-phenanthroline complexes:[Phen2M]2+ {M=Mn (II),Fe(II) Co(II),Ni(II),Cu (II)}; [Phen2M]3+{M=Ti(III),V(III),Cr(III)} as accurate computations of parameters of these techniques[19-23]had become possible by DFT[24,25] only recently .
(b) ν (CN) would be lowered on coordination of N to a metal ion while the transfer of electronic charge from molecular orbitals mainly with metal character to those having the ligand character should increase ν (CN).Since ν (CN) was metal sensitive only to a limited extent, it would be difficult to assign exact value to ν(CN) as many 1, 10-phenanthroline vibrations also appeared in the same region [26]*.Also the π→ π* transition of the ligand and MLCT in some complexes [27]**would absorb in the same region[19], the reflectance spectral technique might not help. Thus the NMR technique was tried which also enabled us to calculate the Spin Hamiltonian (H^) values of these complexes
Methodology
Use of ADF (Amsterdam Density Functional) software to calculate various NMR (29-34), IR (28), ESR (35-39) and NQR (35-39) –parameters had, already, been reported.
Results
Tables: 1-3 represented energies of metals, their gn and I values, thermal and optimization parameters of complexes. Table: 4 gave σ and δ values of 14N, 13C and 1H of the free1, 10-phenanthroline .Table:5 contained σ and δ values of M +n, 14N, 13C and 1H of complexes. Table: 6 contained contributions of 2 diamagnetic, 4 paramagnetic and 4 spin orbit terms (10 in all) in σ of Mn+,14N, 13C, 1H of complexes .Table: 7 gave ESR and NQR parameter of the various types of H, C and N in complexes.Table:8 gave some more ESR and NQR parameters of complexes. Table: 9 contained contributions from five factors into HSpin and ΔEh f values. Table: 10 designated IR-active bands in the complexes. Table: 11 gave σ 14N values of the uncoordinated ligandand the complexes.
Table 1: Energies (kJmol-1) of First Transition Metals
| M | Sum of orbital energies | Total energy | Kinetic energy | Nuclear attraction energy | Electron repulsion energy | Exchange energy |
| Ti | – 49034.160 | – 82193.590 | 83068.461 | -195833.367 | 34453.298 | – 3879.077 |
| V | – 54453.277 | – 91394.073 | 92457.572 | – 218055.74 | 3 8338.78 | – 4184.954 |
| Cr | – 60139.891 | – 101188.38 | 102462.98 | – 241698.34 | 42549.246 | – 4503.221 |
| Mn | -18580.3096 | -31364.4439 | 31791.632 | -75034.4123 | 13237.036 | -1358.702 |
| Fe | -20333.522 | -344593.341 | 349652.94 | -82578.841 | 14339.914 | -1455.918 |
| Co | -78854.221 | – 134234.27 | 136350.81 | – 346177.79 | 57226.702 | – 5539.960 |
| Ni | -85650.855 | -146522.795 | 148997.80 | -352449.098 | 62843.648 | -8809.705 |
| Cu | – 9309.418 | – 159479.16 | 162355.29 | -384859.801 | 69353.856 | – 6328.501 |
Table 2: Optimization Parameters (kJmol-1), [M gn], {MI }and (MS) of [Phen2 M] n + Complexes
| M n+ (D2d) | [M gn]{M I}(M S) | Total bondingenergy** | Total Energy(X c)LDA(Exchange; Correlation)GGA(Exchange; Correlation)a | Nucleus |
| Ti(III) a | [-0.315392]{2.5}(0.5) | -25980.97 | -511912.56-442448.48, -34582.43-52792.02, 17910.37 | 47Ti |
| V(III) | [1.4710588]{3.5}(1.0) | -28816.33 | -484494.18-449671.64, -34822.54 | 51V |
| Cr(III) a | [-0.316360]{1.5}(1.5) | -26138.48 | -529520.63-458827.75, -35164.23-53626.90, 18098.25 | 56Cr |
| Mn(II) | [1.387488]{2.5}(2.5) | -30145.34 | -501580.82-466197.13, -35383.70 | 55Mn |
| Fe(II) | [0.181246]{0.5}(2.0) | -29988.39 | -510676.41-475004.40, -35672.01 | 57Fe |
| Co(II) | [1.322000]{3.5}(1.5) | -29955.67 | -520137.94-484172.74, -35965.19 | 59Co |
| Ni(II) | [-0.500133]{1.5}(1.0) | -29673.45 | -530709.68-494411.25, -36298.43 | 61Ni |
| Cu(II) | [-1.4821933]{1.5}(0.5) | -29439.21 | -540776.87-504181.31, -36595.56 | 63Cu |
* Contains LDA and GGA;a GGA contributes** Energy difference between molecule and fragments..
Table 3: Thermal Parameters of [Phen2 M] n+ Complexes
| [M] n+ | Zero Point Energy(e V) | Thermal Parameters | |||||||||||
| Entropy (cal mol-1K-1) | Internal Energy (Kcal mol-1) | Constant Volume Capacity (Kcal mol-1K-1) | |||||||||||
| Trans. | Rot. | Vib. | Total | Trans. | Rot. | Vib. | Total | Trans. | Rot. | Vib. | Total | ||
| Ti(III) | 9.2711 | 43.91 | 33.1 | 54.3 | 131.26 | 0.889 | 0.889 | 223.3 | 225.1 | 2.981 | 2.981 | 73.88 | 79.84 |
| V(III) | 9.246 | 43.93 | -do- | 49.0 | 126.00 | -do- | -do- | 222.3 | 224.1 | -do- | -do- | 74.43 | 80.39 |
| Cr(III) | 9.2647 | 43.94 | -do- | 51.2 | 128.21 | -do- | -do- | 222.9 | 224.7 | -do- | -do- | 74.03 | 80.0 |
| Mn(II) | 9.256 | 43.96 | -do- | 56.4 | 133.38 | -do- | -do- | 223.5 | 225.2 | -do- | -do- | 77.97 | 83.93 |
| Fe(II) | 9.277 | 43.97 | -do- | 70.4 | 147.44 | -do- | -do- | 225.4 | 227.2 | -do- | -do- | 83.66 | 89.62 |
| Co(II) | 9.244 | 43.99 | -do- | 63.3 | 140.34 | -do- | -do- | 223.8 | 225.6 | -do- | -do- | 80.3 | 86.3 |
| Ni(II) | 9.222 | 43.98 | -do- | 60.7 | 137.74 | -do- | -do- | 223.0 | 224.7 | -do- | -do- | 78.68 | 84.64 |
| Cu(II) | 9.294 | 44.02 | -do- | 64.1 | 141.21 | -do- | -do- | 225.2 | 227.0 | -do- | -do- | 81.34 | 87.30 |
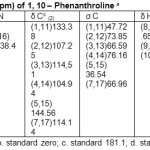 |
Table 4: σ N, σ H, δ N and δ H(ppm) of 1, 10– Phenanthroline a Click here to View table |
![Table 5: σ and δ values (ppm) of M +n , N, C, H in [Phen2M] n+ With M n+ Complexes a](http://www.orientjchem.org/wp-content/uploads/2017/08/Vol33No4_NMR_Seh_tab5-150x150.jpg) |
Table 5: σ and δ values (ppm) of M +n , N, C, H in [Phen2M] n+ With M n+ Complexes a Click here to View table |
![Table 6: Diamagnetic, Paramagnetic & Spin orbit contributions in σ (ppm) of [Phen2M] n+ Complexes a](http://www.orientjchem.org/wp-content/uploads/2017/08/Vol33No4_NMR_Seh_tab6-150x150.jpg) |
Table 6: Diamagnetic, Paramagnetic & Spin orbit contributions in σ (ppm) of [Phen2M] n+ Complexes a |
Table 7: ESR and NQR Parameters of N, C and H from Software for [Phen2 M] n+ Complexes
| M n+ M n+ | gn. A ten values of4 types of H; eachtype having 4H | gn.A ten valueof each oneof 4 N | gn. A ten values of6 types of C; eachtype having 4 C | h values of 6 types of C;each type having 4 C | NQCC andh values ofeach N |
| Ti(III) | 0.776, 0.898,-0.087 -0.092 | -7.654 | 3.109, -0.070, 0.05,1.994,18.360, 0.197 | 0.222,0.251,0.137,0.411,0.680,0.336 | -1.786(0.969) |
| V(III) | 0.244, 0.572,0.465,0.118 | -6.862 | 6.118, 0.057, 0.863,0.573, 8.492, 0.317 | 0.394,0.267.0.141,0.443,0.617,0.339 | 1.621(0.558 ) |
| Cr(III) | 0.115, -0.324,-0.70, 0.825 | -4.715 | 3.261, 0.059, 0.08,3.429, 0.207, 0.795 | 0.345,0.250,0.139,0.465,0.606,0.332 | 1.511( 0.464) |
| Mn(II) | -1.233, 0.629,-0.333, 0.88 | 2.184 | 1.125, -0.357, 0.436,0.313, 3.512, 0.122 | 0.104,0.150,0.080,0.256,0.597,0.229 | -2.112(0.635 ) |
| Fe(II) | -0.962, 0.677,0.247, 0.152 | 5.221 | 0.831, -0.106, 0.314,0.553, 2.012, 0.118 | 0.038,0.154,0.077,0.247,0.683,0.230 | -2.073(0.597) |
| Co(II) | -0.895, 0.928,0.913, 0.299 | 9.623 | 0.857, 0.188, 0.292,0.95, 1.622, 0.158 | 0.092,0.160,0.078,0.262,0.665,0.231 | -1.893(0.698) |
| Ni(II) | -1.191, 1.907,2.324, 0.466 | 16.892 | -2.322, 0.146, 0.321,1.922, 2.661, 0.015 | 0.056,0.155,0.077,0.276,0.661,0.230 | -1.807(0.710) |
| Cu(II) | -1.686, 3.080,2.934, -0.62 | 19.753 | -2.992, 0.349, 0.372,2.06, 5.007, -0.866 | 0.032,0.144,0.076,0.261,0.679,0.211 | -1.959(0.581) |
![Table 8: ESR and NQR Parameters* of M +n from Software* for [Phen2 M] n+ Complexes](http://www.orientjchem.org/wp-content/uploads/2017/08/Vol33No4_NMR_Seh_tab8-150x150.jpg) |
Table 8: ESR and NQR Parameters* of M +n from Software* for [Phen2 M] n+ Complexes |
![Table 9: Calculation of H^ and Eh f Parameters of [Phen2 M] n+ Complexes](http://www.orientjchem.org/wp-content/uploads/2017/08/Vol33No4_NMR_Seh_tab9-150x150.jpg) |
Table 9: Calculation of H^ and Eh f Parameters of [Phen2 M] n+ Complexes |
Table: 10. Designations of IR Active Bands in [Phen2 M] n+ Complexes
| Complex with M +n | Vibration Symmetry of bands* | IR active bands | Vibration Symmetry Class |
| Ti(III),V(III),Cr(III), Mn(II),Fe(II), Co(II),Ni(II),Cu(II) | A(129) | A(129) | [129A] |
*Numbers in parentheses indicate bands of a specific symmetry
Table: 11. σ N values of 1, 10-Phenanthroline and its complexes with metal ions
| σ values | Phen | Ti(III) | V(III) | Cr(III) | Mn(II) | Fe(II) | Co(II) | Ni(II) | Cu(II) |
| σN | -138.4 | 316.50 | 296.30 | 310.20 | 178.39 | 140.68 | 84.80 | -1.15 | -163.1 |
![Figure 1: [Phen2M]n+{M=Ti (III),V(III), Cr(III),Mn (II),Fe(II), Co(II),Ni(II),Cu(II) at:1}](http://www.orientjchem.org/wp-content/uploads/2017/08/Vol33No4_NMR_Seh_fig1-150x150.jpg) |
Figure 1: [Phen2M]n+{M=Ti (III),V(III), Cr(III),Mn (II),Fe(II), Co(II),Ni(II),Cu(II) at:1} |
Discussion
Each one of the eight 1, 10-phenanthroline complexes contained 45 atoms:
4 N,16 H, 24C and a different transition metal ion.
Relations Used to Calculate NMR Parameters [40]
(a) σ M n+,σ1H,σ13C and σ14N were equal to the sum of values of 2 diamagnetic, 4 paramagnetic and 4 spin orbit terms of M n+, 1H , 13C and 14N respectively.
(b) (σ) and (δ) of 1H , 13 C and 14 N were related as follows:
δ1H = 31.7 – σ 1H —————————————————————— (1)
δ13C=181.1- σ 13C—————————————————————— (2)
σ M = – δ M |
σ17O= – δ 14N ——————————————— (3)
Relations Used to Calculate ESR Parameters [35-37]
Effective Spin Hamiltonian (H^) [35-37]
No doubt, three relations [35-39] were needed to calculate H^ but all the 8 complexes included in the present study were axially asymmetric having the same two g values (g⊥) while the third g of different value and was represented as g ll .Correspondingly, two same a values ( a⊥) while the third a of different value was named as a ll , only the following relation would suffice:
H^ = be [gll .H ll. S +g⊥(2H⊥. S)] + [a ll . S .I +a⊥(2S.I)] +Q [I -1/3 I (I+1)] + D {Sz2-S(S+1)/3} – [gn.bn.H0 .I] —– (4)
Five factors: g factor, a factor, Q factor, Zero Field Splitting (ZFS) factor [41,42] and interaction of nuclear magnetic moment with external magnetic field, i.e. I factor would contribute in the total value of Spin Hamiltonian (H^). Sz representing spin angular momentum was calculated as:
Sz = S/S(S+1)0.5 ———————————————- (5)
Hyperfine Coupling Energy [41]
ΔE hf = 1/2[a112 +a222 + a332]1/2 ———————————— (6)
(c) H^ values were calculated both in terms of MHz as well as in joules mol1-.
Their inter conversions were given as:
(i) One MHz=6.627*10-21 erg = 3.9903124*10-7 kJ mol-1
(ii)1cm-1= 0.0119626kJ mol-1 = 29979.2458 MHz
(iii) For 8388.255 MHz in a 0.30T, the g value of the standard substance: 2, 2-diphenyl-1-picrylhydrazyl (DPPH) was: g DPPH 2.00232[43-45].So g value of the complex (gM n+) and its frequency (νM n+) were related as follows:
νM n+ =8388.255 * g M n+ /2.00232———————— (7)
The details of the various terms involved were discussed elsewhere [28].
Relations Used to Calculate NQR Parameters [46, 47]
Asymmetry Coefficient (h) [46, 47]
h=q xx – q y y /q z z ———————————————– (8)
(h) lies in between 0-1.For axial symmetry, h=0. It was possible only when:
qx x =q y y ¹q z z ————————————————– (9)
Laplace Equation [46, 47]
qx x + q y y +qz z =0 ———————————————-(10)
Calculation of four NMR and NQR Parameters
Four more ESR and NQR parameters: H^, ΔE hf,η , and Laplace equation which were calculated in addition to five ESR(g11, g22, g33, g iso ; a11, a22, a33, A ten) and NQR parameters (η; q11, q22, q33; NQCC) parameters obtained from the software for 7 complexes excluding [Phen2Fe] 2+where ADF software did not work. All the 7complexes possessed axial symmetry with (a) Two of three g called g⊥ were of the same value and third of higher value was called g|| (b) Two “a” called a⊥ were of the same value and the third of higher value was called a11. (c) Two of the three q parameters were of the same value. (d) η=0. Relation (4) was applicable to all to calculate H^. Individual values of these five factors in the total value of H^ were given at bottom and were represented as (→). ΔE hf, η, Laplace equation were calculated by relations (7, 8,10) respectively in Tables:7-9.NQCC, a, q were expressed in MHz while g was unit less.
Confirmation of Spatial Equivalence of N, C, H In Complexes
It was confirmed by following two ways:
From the Equivalence of NMR Parameters
For ascertaining the stereochemistry of the complexes, the 4 coordinating N, the 24 C and the 16 H were classified according to their spatial displacements. The metal ion formed a class of its own. The spatially equivalent species possessed same values of δ, σ along with each one of the ten contributing terms towards the total value of σ of the constituents. δ M n+,δ14N, δ 13C ,δ 1H, σ M n+, σ 14N, σ 13C,σ 1H and 10 contributing terms of all the 45 species in each one of the 8 complexes were reported(Tables:5,6). All the four coordinating 14N in each complex were spatially equivalent with the same value each for σ14N, δ14N and the 10 contributing terms respectively. Each complex having 16 1H contained four types of stereo chemically different 1H; each type possessing four equivalent protons as they showed four different series of values σ1H, δ1H and 10 contributing terms respectively. The 24 13C of each complex contained 6 types of spatially different 13C; each type having four equivalents 13C as they gave six different series of values of σ13C and δ13C and contributing 10 terms respectively.
From Equivalence Among five NMR, ESR, NQR Parameters
A total of five parameters of three techniques [ESR(A ten), NQR (NQCC,η) and NMR (σ, δ)] were used to ascertain similar stereochemistry of 8 paramagnetic complexes. Four stereo chemically equivalent 14N possessed the same values of above named five parameters. The four parameters: gn. A ten, η, σ, δ of 24 13C were categorized into 6 types (NQCC=0 for all); each having 4 equivalents C. Three parameters (gn. A ten, σ, δ) of the 16 H were of 4 types (NQCC and η =0 for all); each type having 4 equivalents 1H. So the three techniques corroborated to confirm that each one of these complexes possess one type of 4 N; six types of 24 C and four types of 16H. Further, the two NMR parameters (σ, δ) of constituents were shown in Table: 5 and the remaining three [one of EPR (A ten) and two of NQR (NQCC,η ) were shown in (Table: 7). Again, with I=1/2 for 1H and 13C; their NQCC =0.0.This left 4 parameters each for1H and 13C.All the η values were zero for all the 16 1H.So three parameters were reported for 1H.
Evidence of MLCT Phenomenon from NMR Parameters of Complexes
MLCT phenomenon would increase an electron density on the ligand by shifting electronic charge from the filled molecular orbitals of complex lying mainly on metal to its vacant molecular orbitals with energies comparable to the ligand. As σ of a nucleus was directly related to its electron density, any change in its value should serve as an indicator to the change in electron density on it. In 7 of the 8 complexes {except in [Phen2Cu] 2+}, total shielding tensors of the σ14N,σ 13C, σ1H (Tables:11,5) were observed to be higher and the δ14N, δ14C, δ1H were found to be lower than their values in the uncoordinated ligand (Table:4).It confirmed the transfer of electronic charge from metal to the ligand and thereby the MLCT.
IR Parameters of Complexes
The software gave values of frequencies, dipole strengths and absorption intensities of IR-active normal modes of all the 129 fundamental vibration bands of the complexes; each having A (singly degenerate ) symmetry according to their IR- activities [48] with a Vibration Symmetry Class {129A} (Table: 10).
Conclusions
With higher σ14N, σ13C, σ1H and lower δ14N, δ14C, δ1H values of constituents in these complexes relative to uncoordinated ligand would confirm an increase in the electron density on the ligand in the complexes to lend support to the transfer of electron cloud from the metal into the ligand orbitals as per MLCT definition.
NMR, ESR and NQR results corroborated with one another to prove that all these 8 complexes possessed the same stereochemistry as all the 44 atoms were occupying the same relative positions around each one of metal ion.
References
- Satar, A.; Jabbar, A,; Rzokee, Ahmad, A. Int. J. Basic App. Chem. Sci. 2014, 4, 37-50.
- Zalis, S.; Consani, C.; El Nahhas, A.; Canizzo, A.; Chergui, M.; Hart, F.; Vlecek, A. Inorg. Chim. Acta. 2011, 374, 578- 85
CrossRef - Jesse, V. G.; Christopher, J. E.; Lev, N. Z.; Mattew, E. C.; Michael, M.H.; Darren, W. J. Chem. Sci. 2014, 5, 2899-2905.
CrossRef - Yutaka, T.; Obara, S.; Ogawa, S.; Nozaki, K.; Ikeda, N.; Ohno, T.; Ishii, Y.; Sakai, K.; Aski, M. H. Inorg Chem. 2005, 44, 4737-46.
CrossRef - Mudasira, K. W.; Daryono, H. T.; Naoki, Y.; Hidenari, I. Z. Naturforsch. 2004, 59, 310-18.
- Sreekanth, B.; Krishnamurthy, G.; Bhojya N. H. S.; Vishnuvardhan, T. K.; Shashikumar, N. D.; Lokesh, M.R. J. Chem. Pharm. Res. 2011 , 3 , 407-19.
- Sreekanth, B.; Krishnamurthy, G.; Naik, H.S.; Vishnuvardhan, T.K.; Vinaykumar, B. S. N. Nucleosides Nucleotides Nuc. Acids. 2011, 30, 83-96.
CrossRef - Suescun, L.; Mombru, A.; Mariezcurrena, R.A.; Baggio, R. Acta. Cryst. C. 2000, 56, 179-81.
CrossRef - Scaringe, R.; Singh, P,; Eckberg, R.P.; Hatfield, W.E.; Hodson, D.J. Inorganic Chem. 1975, 14, 1127-33.
CrossRef - Yesilel, O.; Olmez, H. J. Thermal Analy. Calorimet. 2006, 86, DOI: 10.1007/s10973-005-7137-2
CrossRef - Chis, V.; Droghetti, A.; Isai, R.; Morari, C., Rungger, I.; Sanvito, S. AIP Conf. Proc. 2013, 57; 25-27.
- Ozel, A.E.; Kecel, S.; Akyuz,S. Mol.Str. 2007, 834, 548-54.
CrossRef - Morigakii, M.K.; Elias, M. D.S.; Carlos, V.P. D.M.; Jamile, R.P.; Renzo, C.S.; Armando, B.; Jair, C.C.F.; Gilson, H.M.D. Quim.Nova 2009, 32.CrossRef
- Chiniforoshan, H.Z.; Sadeghian, L.; Tabrizia, H.; Tavakol, M.; Sabzalian, G.; Mohammad nezhad, H.; Goris, W.P. Mol. Str. 2015, 1081, 237-43.
CrossRef - Sanotra, S.; Gupta, R.; Gupta, U.; Khajuria, Y. Spectrochim. Acta. Part-A. 2014, 129, 392-99.
CrossRef - Euan, K. B.; Lucia, C.; Ulli, E.; Ludovica, M.; Guido, P.; Calogero, P.; Alessanodro, P. Inorg. Chim. Acta. 2008, 361, 2375- 84.
CrossRef - Panina, N.S.; Demidov, V. N.; Simanova, S. A. Russian J. Gen. Chem. 2008, 78, 919-24.
CrossRef - Zn, B.; Xiao, Y. Prog. zeolite microporous mat. PTC a-c. 1997, 105, 615-22.
- Kaupp, M.; Malkin, V.G.; Malkina, O.L. Encyclo. Computat. Chem. 1998, 1857-66.
- Schreekenbatch, G.; Zieglar, T. Theo. Chem. Acc. 1998, 99, 71-78.
CrossRef - M. Buhl, M. Kaupp, O.G. Malkina , V.G. Malkin, “The DFT route to NMR chemical shifts,” J. Comput.Chem. 1999,20(1), 91-105
CrossRef - Autschbach, J.; Zieglar, T. Coord. Chem. Rev. 2003, 238/239, 83-126.
CrossRef - Buhl, M. Theo. App. 2004, 421-31.
- Autschbach, J.; Zieglar, T. Encyclo Nuc. Mag. Reson. 2002, 9, 306-23.
- Buhl, M.; Mauschick, F.T. Phys. Chem. Chem. Phys. 2002, 4, 5508-14.
CrossRef - Brahham, S.; Sachin, H.P.; Shivshankar, S.A.; Narsimhamurthy, T.; Rathore, R. M. Acta Crys. C. 2008, 64, 140 -143.
- Schilt, A. A.; Taylor, R. C. J. Inorg. Nuc. Chem. 1959, 9, 211–21.
CrossRef - Sehgal, M.L.; Ahmad, I. Oriental J. Chem. 2017, 33.
- Sharma, S.; Chander, S.; Sehgal, M.L.; Ahmad, I. Oriental J. Chem. 2015, 31, 1417-27.
CrossRef - Singh, H.; Bhardwaj, A.K.; Sehgal, M.L.; Ahmad, I. Oriental J. Chem. 2015, 31, 671-79.
CrossRef - Singh, H.; Bhardwaj, A.K.; Sehgal, M.L.; Javed, M.; Ahmad, I. Oriental J. Chem. 2015, 31, 1461-68.
CrossRef - Schreckenbach, G.; Ziegler, T. J. Phys. Chem. 1995, 99, 606-11.
CrossRef - Schreckenbach, G.; Ziegler, T. Int. J.Quantum Chem. 1997, 61, 899-918.
CrossRef - Wolff, S.K.; Ziegler, T. J. Chem. Phys. 1998, 109, 895.
CrossRef - Singh, H.; Bhardwaj, A.K.; Sehgal, M.L.; Susheel, K.M. Int. J. Curr. Res. Rev. 2012, 4, 12-28.
- Singh, H.; Bhardwaj, A.K.; Sehgal, M.L.; Susheel, K.M. Int. J. Curr. Res. Rev. 2013, 5, 13-31.
- Singh, H.; Bhardwaj, A.K.; Sehgal, M.L.; Susheel, K.M. Int. J. Curr. Res. Rev. 2013, 5, 71-88.
- Neese, F. Chem. Phys. 2007, 127, 164112.
- Schmitt, S.; Jost, P.; Van Wullen, C. J. Chem. Phys. 2011, 134, 194113.
CrossRef - Autschbach, J. Str. Bond. 2004, 112, 1-43.
CrossRef - Russel, D.S. Phy. methods in Chem. 1977, 227, 361-97.
- Sandberg, K.A. Ph.D.Thesis: Chemistry Department, NC State University,1998.
- Abragam, A.; Bleaney, B.Electron Paramagnetic Res. Trans. Ions. 1986.
- Schweiger, A.; Jeschke, G. Prin. Pulse Elec. Paramagnetic Res. 2001
- Weil, J. A.; Olton, J.R. Elec. Paramagnetic Res. Spectro. Elem. Theo. Applicat. 2007.
- Edmonds, D.T.; et al. Adv. Quadrup. Res. 1974, 1, 145.
- Smith, A. S. Adv. Nuc. Quadrup. Res. 1975, 2, 1977, 3.
- Nakamoto, K. Infrared Raman. Spec. Inorg. Coord. Comp. 1986, 291-98.

This work is licensed under a Creative Commons Attribution 4.0 International License.









