Cytotoxicity Effects of Extracts and Essential oil of Kaempferiagalanga on Cervical Cancer C33A Cell Line
Muhammad Nor Omar1, S. M. M. Abdul Rahman1, S. J. A. Ichwan2, N. H. M. Hasali1, Faridah Abdul Rasid1 and Farhana Abdul Halim1
1Kulliyyah of Science, International Islamic University Malaysia, 25200 Kuantan Pahang Malaysia.
2Kulliyyah of Dentistry, International Islamic University Malaysia, 25200 Kuantan Pahang Malaysia.
Corresponding Author E-mail: mnoromar@iium.edu.my
DOI : http://dx.doi.org/10.13005/ojc/330409
The cytotoxicity properties of Kaempferiagalanga extracts were studied. Crude extracts of the rhizome were obtained by extraction with methanol (KGM), petroleum ether (KGPE) and ethyl acetate (KGEA). Meanwhile, the essential oil (KGEO) was produced by steam distillation method. The aim of the present work was to screen the cytotoxic activity of K. galangaextracts and essential oil on cervical cancer C33A cell line. The cytotoxic activities were assessed on C33A cell line using MTT and scratch assays. Results of MTT assay showed that KGEA and KGM were the most cytotoxic at 1000 µg mL-1 with cell viabilities of 11 and 14 %, respectively. Lower cytotoxic activities were shown by KGPE and KGEO with 64 and 88 % cell viability, respectively. Meanwhile, KGEA was slightly cytotoxic at concentration of 500 µg mL-1 with cell viability of 70 %. However, all the extracts were able to inhibit or at least slow down cell growth when tested using the scratch assay. It can be concluded that K. galanga seems to have cytotoxic properties and may be used as an anticancer agent.
KEYWORDS:Kaempferiagalanga; cytotoxic; cervical cancer C33A cell line
Download this article as:| Copy the following to cite this article: Omar M. N, Rahman S. M. M. A, Ichwan S. J. A, Hasali N. H. M, Rasid F. A, Halim F. A. Cytotoxicity Effects of Extracts and Essential oil of Kaempferiagalanga on Cervical Cancer C33A Cell Line. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Omar M. N, Rahman S. M. M. A, Ichwan S. J. A, Hasali N. H. M, Rasid F. A, Halim F. A. Cytotoxicity Effects of Extracts and Essential oil of Kaempferiagalanga on Cervical Cancer C33A Cell Line. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=34809 |
Introduction
Malaysian medicinal plants have been screened extensively due to their pharmaceutical properties. These include plant species fromZingiberaceae family such as Alpinia1, Zingiber2,3, Galanga3 and Kaempferia4,5. Besides these species, other plants from Artocarpus6,7, Andrographis8-10, Centella11, Citrus12,13, Cymbopogon14, Plumeria15 and Musa16,17 have been assessed, especially for their biological activities.
Cytotoxicity studies of Zingiberaceae plant extracts, especially on cancer cell lines have been evaluated. Recently, Ibrahim18 showed that the bioactive principal from Z zerumbet, i.e. zerumbone inhibits the proliferation of human cervical cancer cell line. Meanwhile, the methanolic extract of the K. galanga rhizome contains ethyl-p-methoxycinnamate, which is highly cytotoxic to HeLa cells19, besides showing larvicidal activity against second stage dog roundworm larva, Toxocara canis20. Ethyl p-methoxycinnamate has been reported to have many biological properties such as anticancer and anti-monoamine oxidase activities21,22. K. galangaextracts have potent nematicidal effects as well23. A considerable amount of work has been done for the isolation and pharmacological evaluation of different constituents from K. galangaextracts. The larvicidal, nematicidal, vasorelaxant, and antineoplastic effects of the herb are mainly due to ethyl cinnamte and ethyl-p-methoxycinnamate24. Recently, Jagadishet al25 reported that successive ethyl acetate extract of K. galangashowed selective toxicity against four types of cancer cells. Meanwhile, successive petroleum ether extract showed selective toxicity against certain cells only. The other extracts were cytotoxic only when the cultures were exposed to very high concentrations of extract.
Materials and Method
Chemicals for Cell Culture
Phosphate buffer saline (PBS, GIBCO), Dulbecco’s Modified Eagle Medium (DMEM, GIBCO), penicillin-streptomycin (GIBCO), fetal bovine serum (FBS, Sigma-Aldrich), trypsin solution (GIBCO), Thiazolyl blue tetrazolium bromide (MTT) stock solution (Sigma-Aldrich), hematoxyline solution (Sigma-Aldrich) and 90 % methanol were used for cell culture.
Cell line
Cell line representing cervical cancer, C33A, were obtained from Department of Molecular Craniofacial Embryology, Tokyo Medical and Dental University by the courtesy of Prof. Masa-Aki Ikeda.
Plant Materials
K. galanga rhizomes were bought from the local wet market in Kuantan, Malaysia. The rhizomes were washed and sliced before drying in a vacuum oven (Memmert, Manchester) at 45 °C for five days until the samples were completely dry. Then, the samples were ground using a blender and stored at – 4 °C prior to further analyses.
Extraction Procedure
The powdered rhizome (250 g) was extracted with ethanol (300 mL) in a Soxhlet extractor for 20 h. The extract was concentrated using a rotary evaporator (Buchi, Switzerland) under reduced pressure and controlled temperature (50 °C) to afford a brownish-black coloured semi-solid residue denoted KGM (13.44 %). In another extraction, the powdered rhizome (250 g) was extracted successively in a Soxhlet extractor with petroleum ether (60 – 80 °C) and ethyl acetate for 20 h. The extracts were also concentrated using a rotary vacuum evaporator under reduced pressure and controlled temperature. The petroleum ether extract yielded a golden yellowish oily liquid, KGPE (1.38 %) and the ethyl acetate extract yielded a dark brownish semi solid residue, KGEA (1.67 %). Meanwhile, K. galanga essential oil was prepared from powdered rhizome using steam distillation method. This afforded the essential oil, KGEO (4.53 %). Accurately weighed extracts were dissolved separately in distilled dimethyl sulfoxide (DMSO) and the volume was made up to 10 mL with DMEM in 2 % FBS to obtain a stock solution of 1 mg mL-1 concentration. The mixtures were stored at – 20 °C until further use.
Cytotoxicity Assay
Cell Lines and Culture Medium
The cells were maintained in DMEM containing 2 % FBS and grown in culturemediumuntil confluent25-27. Cells were observed under an inverted microscope to check for 90 % confluence. The flask was shaken to suspend the dead cells and washed using PBS to remove excess FBS. Then, 1 mL of trypsin was added with shaking and then incubated at 37 °C in 5 % CO2 for three minutes to detach the cells. Once the mixture was turbid, 4 mL of DMEM was added. After shaking and mixing, the contents of the flask were divided equally and the volumes of the new petri dishes were made up to 5 mL using DMEM. The cells were incubated for 48 hours at 37°C in 5 % CO2.
MTT Assay and Minimum Inhibitory Concentration (MIC)
The MIC determination was done by using the broth micro dilution method and performed using sterile 96-well round micro-plates. The sample concentrations used were between 1000-125 µg mL-1 using two-fold serial dilutions. Samples were added to the wells keeping the DMSO in each well at 0.2 % maximum. For a final concentration of 1000 µg mL-1, 80 µL KGPE, KGEA and KGEO extracts were added to 720 µL DMEM. But for the KGM extract, 14 µL extract was added to 786 µLDMEM as more DMSO was needed to dissolve the KGM. From the 1000 µg mL-1 solution, the samples were diluted using two-fold serial dilutions a total of three times. The samples were added to each well except the control wells which were filled with either MTT, DMSO or DMEM. This was followed by incubation at 37 °C for 24 h. After 24 h, MTT solution was added to the wells and left for 1 h. The change of wells from yellow to purple colour indicates that the cancer cells were alive while those with no change indicate a constriction in cell growth. The plate was read using a 96-well micro-plate reader to further analyze cell viability in detail.
Scratch Assay
Cells were cultured in a 12-well microplate until cells reached approximately 90 % confluence. The cell monolayer was scraped with a p200 pipette tip in a straight line to create a scratch. Scratches of approximately similar sizes were made in the assessed cells and control cells to minimize any possible variation caused by the difference in width of the scratches. The old medium was discarded and then replaced with 5 mL of the new medium mixed with the extracts. To obtain the same field during image acquisition, markings to be used as reference points were created close to the scratch. The reference points were made by etching the dish lightly with a razor blade on the outer bottom of the dish or with an ultrafine tip marker. After the reference points were made, the dish was placed under a phase contrast microscope, and the reference mark was left outside the capture image field but within the eye-piece field of view. A first image of the scratch was acquired. The dish was placed in a tissue culture incubator at 37 °C for 8-18 h. The dishes were taken out of the incubator to be examined periodically and thenreturned to resume incubation. After incubation, the dishes were placed under a phase-contrast microscope, the reference point matched, the photographed region acquired previously was aligned and a second image was recorded28. The images acquired for each sample was analyzed by measuring and comparing the width of the gap until the control gap completely closed up.
Results and Discussion
Table 1: MTT assay of different extract concentrations on C33A cell viability (%)
|
Cell Viability % |
|||||||
|
Concentration (mgmL-1) |
KGEA |
KGM |
KGEO |
KGPE |
DMSO | DMEM | |
|
1000 |
11 |
14 |
88 |
64 |
100 | 100 | |
|
500 |
70 |
100 |
100 |
100 |
|||
|
250 |
100 |
100 |
100 |
100 |
|||
|
125 |
100 |
100 |
100 |
100 |
|||
|
0 |
100 |
100 |
100 |
100 |
|||
MTT Assay and Minimum Inhibitory Concentration (MIC).
Table 1 shows the percentage of cell viability when different concentrations of the samples were added to the medium. Cell viability was read using a microplate reader. Figure 1is the graph for the half maximal inhibitory concentration (IC50) of the samples against the C33A cell line. The IC50 value is the concentration of sample when cell viability was at 50 %. For KGEA, the IC50 was between 600 and 700 µg mL-1 while for KGM it was 800 µg mL-1. The IC50 for KGPE and KGEO were estimated by extending the lines of the two samples. The estimated IC50 value for KGPE was between 1100 and 1200 µg mL-1 and the estimated IC50 value for KGEO was 2000 µg mL-1. MTT was carried out to test the cytotoxicity of the extracts. This assay was carried out simultaneously as MIC as the same conditions were used. MIC was carried out to determine the minimum concentration of extract that could inhibit cell growth, or in this case cause cell viability to decrease. KGEA and KGM were the most cytotoxic at 1000 µg mL-1 where less than 15 % of the cells were viable. KGEO and KGPE showed some cytotoxicity at the same concentration but only KGEA was slightly cytotoxic at 500 µg mL-1. DMSO was included in the assay as a control. From the results, the cells incubated with DMSO were 100 % viable, which means the solvent was not cytotoxic to the cells. The half maximal inhibitory concentration (IC50) which is the measure of the effectiveness of a compound in inhibiting biological or biochemical function was calculated. The readings were taken by measuring the concentration of the sample when the cell viability was at 50 %. By calculating the IC50, the most cytotoxic sample and the concentration where approximately 50 % of the cells would be killed was identified. From the results (Figure 1), it can be said that KGEA is most cytotoxic followed by KGM, KGPE and KGEO since the IC50 value for KGEA was the lowest (600 µg mL-1).
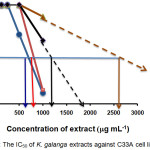 |
Figure 1: The IC50 of K. galanga extracts against C33A cell line
|
Scratch Assay
Figure 2 shows how the KGEA extract at concentration 500 µg mL-1 influenced scratch size. Images taken on the first, second, fourth, sixth and eighth day were compared. From the second day to the fourth day, the size of the scratch decreased only slightly and remained the same till day eight. However, by the last day, cells were attaching to the scratch region.
For the 2-fold dilution of the same extract (Figure 3), the scratch size decreased slightly on the second day. From the fourth day onwards, another spot was used and over the remaining four days, there was no decrease in scratch size. However, similarly, cells started to attach in the scratch region.
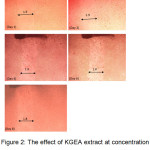 |
Figure 2: The effect of KGEA extract at concentration 500 μgmL-1 on the scratch closing of the C33A cell line from day 1 to day 8 |
The KGM extractat 500 µg mL-1 concentration applied to the cells led to a decrease in scratch size on the fourth day; thereafter the scratch size remained the same until the sixth day. However, on the eighth day, the scratch size increased slightly. Extract at concentration of 250 µg mL-1 applied to the cells showed a slight increase in the scratch size after two days along with increasing number of cell colonies in the scratch area. KGEO extracts at concentration of 500 µg mL-1 exhibited a steady decrease in the scratch size from the second day until the scratch was not visible on the eight day. However, for the same extract of a lower concentration the size only decreased slightly from the first day to the second day and remained the same till the sixth day.
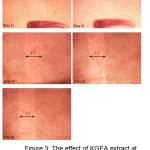 |
Figure 3: The effect of KGEA extract at concentration 250μgmL-1 on the scratch closing of C33A cells from day 1 to day 8 |
The size of the scratch with KGPE extract applied at concentration of 500 µg mL-1 showed approximately no change as the days passed. However, the colonies formed in the scratch zone increased a lot by the sixth day. When the image was captured at another spot after eight days, the scratch zone was still clear of colony formation. The same situation was observed in the C33A cells treated with KGPE at concentration 250 µg mL-1.
The control for each experiment was carried out twice. For the first control DMSO, the scratch size decreased slightly from the first to the second day. Although on the fourth day the scratch area was still visible and seemed to be filled with floating cells, by the sixth day the scratch area still appeared visible. When the microplate was shaken slightly it could be seen that the cells were attached to the surface of the wells and new cells of a lighter shade filled the scratch area. The lighter shade was probably because the cells were not as concentrated at the scratch region yet. However, for the second DMSO control the size of the scratch hardly decreased even after eight days and the cells seemed to clump up at certain spots. This could be due to excessively hard scratching, which resulted in the cells being unable to attach to the dish surface.
For the first DMEM control, the scratch closed up in six days, similar to the first DMSO control. The scratch size decreased considerably in one day and by the fourth day the scratch could hardly been seen. By the sixth day, the scratch was completely gone. For the second DMEM control, the scratch size decrease was slower. On the sixth day, the scratch size only decreased slightly but by the eighth day, it was completely closed up.
Scratch assay was carried out to determine if the extract could be used to retard cell growth or even cell apoptosis. After carrying out the MTT assay it was decided that the concentration of extracts to be added would be 500 and 250 µg mL-1, as a concentration of 1000 µg mL-1 for all the extractscaused cell death and decreased viability. DMEM and DMSO were used as controls. DMEM was used as an indicator to see how long it would take for the scratch to close up without any added extracts. DMSO which was used to dilute the extracts was also used as a control to ensure that the DMSO did not positively nor negatively affect the results of the experiment. Results obtained from the MTT assay showed that even if the cells did not cause cell death, the same concentration could cause growth retardation where the cell growth is completely stopped or is slowed down.
Conclusion
From the MTT assay, it can be concluded that all extracts were cytotoxic at higher concentration; however, KGEA was more cytotoxic at lower concentrations. The results of IC50 indicated that KGEA is the most cytotoxic followed by KGM, KGPE and KGEO. Meanwhile, in the scratch assay, all the extracts retarded and slowed down the scratch closing process. This was related to the MTT assay where the extracts decreased the viability of the cells even at lower concentrations. The MTT results obtained show that even if the cells did not cause cell death, the same concentration could cause growth retardation where the cell growth is completely stopped or is slowed down.
Acknowledgement
Authors would like to thank the International Islamic University Malaysia for the permission to carry out this research under Research Matching Grant Scheme (RMGS10-002-0012).
References
- De Pooter, H.L.; Omar, M.N.;Coolseat, B.A.; Schamp, N.M. Phytochemistry1985,24, 93-96.
CrossRef - Omar, M.N.; Razman, S.; Nor-Nazuha, M.N.;Nazreen, M.N.M.; Zuberdi, A.M. Orient. J. Chem.2013,29, 89-92.
CrossRef - Omar, M.N. J. Trop. Agric. Food Sci.1991,19, 147-152
- Omar, M.N.; Hasali, N.H. M.; Alfarra, H.Y.; Yarmo, M.A.;Zuberdi, A.M. Orient. J. Chem.2014, 30, 1037-1043.
CrossRef - Hasali, N.H.M.; Omar, M.N.;Zuberdi, A.M.;AlFarra, H.Y. Int. J. Biosci. 2013, 3, 148-155.
CrossRef - Kamal, T.; Muzammil, A.; Abdullateef R.A.;Rahma M.S.; Omar, M.N. J. Med. Plants Res. 2012, 6, 4354-4357.
- Jalal, T. K.; Ahmed, I. A.; Mikail, M.; Momand, L.; Draman, S.; Isa, M. L. M.; Bahari, M. S. Appl. Biochem. Biotechnol. 2015,175, 3231-3243.
CrossRef - Sule, A.; Ahmed, Q.U.; Latip, J.; Samah, O.A.; Omar, M.N.; Umar, A.; Dogarai, B.B.S. Pharm. Biol.2012, 50, 850-856.
CrossRef - Sule, A.; Ahmed, Q.U.; Hassan, N.M.; Kamal, L.Z.M.; Samah, O.A.; Omar, M.N.;Yarmo, M.A. Am. J. Appl. Sci.2011,8, 525-534.
CrossRef - Sule, A., Ahmed, Q.U., Samah, O.A. and Omar, M.N. J. Med. Plants Res.2012, 5, 7-14.
- Alfarra, H.Y.; Omar, M.N. Int. J. Pharma Med. Biol. Sci.2014,3, 1-8.
- Omar, M.N. J. Trop. Agric. Food Sci.1999,27, 225-229.
- Omar M.N. J. Trop. Agric. Food Sci.1999,27, 231-236.
- Omar, M.N.; Kasbon, S.N. TeknologiPertanian1981,4, 185-188.
- Nor, M.M.;Hassan, H.H.M.;Ravi, N.H.N.R.;Omar, M.N. J. Agric. Sci. Technol. B.2014, 4, 195-199.
- Mahmood, A.; Omar, M.N.; Ngah, N. Asian Pac. J. Trop. Med.2012,5, 882-886.
CrossRef - Mahmood, A.;Ngah, N.; Omar, M.N. Eur. J. Sci. Res.2010,66, 311-318.
- Ibrahim, A.S. Antitumor effect of zerumbone isolated from lempoyang (Zingiberzerumbet) on human cervical cancer cells and mouse cervical intraepithelial neoplasia.PhD thesis 2009Universiti Putra Malaysia.
- Kosuge, T.; Yokota, M.; Sugiyama, K.; Saito, M.; Iwata, Y.;Nakura, M.; Yamamoto, T. Chem. Pharm. Bull.1985, 33, 5565-5567.
CrossRef - Kiuchi, F.; Nakamura, N.; Tsuda, Y.; Kondo, K.; Yoshimura, H. Chem. a Pharm. Bull.1988,36 , 412 – 415
CrossRef - Zheng, G. Q.; Kenny, P. M.; Lam, L.K.T. J. Agric .Food. Chem. 1993, 41, 153-156.
CrossRef - Noro, T.;Miyase, T.; Kuroyanagi, M.; Ueno, A.; Fukushima, S. Chem. Pharm. Bull. 1983, 31, 2708-2711.
CrossRef - Umar, M .I.;Asmawi, M. Z. B.; Sadikun, A.;Altaf, R.; Iqbal, M. A. Afr. J. Pharm. Pharmacol.2011,5, 1638-1647.
CrossRef - Liu, B.; Liu, F.; Chen, C.;Gao, H. Nat. Prod. Res. 2010,24, 1927-1932.
CrossRef - Jagadish, P.C.; Chandrasekhar, H.R.; Kumar, S.V.; Latha, K.P. Int. J. Pharm. Bio. Sci. 2010,1, 1-5.
- Mosmann, T. J. Immunol. Methods 1983, 65, 55-63.
CrossRef - Omar, M. N.; Hasali, N. H. M.; Yarmo, M. A.Orient. J. Chem. 2016,32, 2731-2734.
CrossRef - Liang, C.C.; Park, A.Y.; Guan J.L. Nat. Protoc.2007,2, 329-333.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









