A Novel Polyester Fabric Coated with Nanoclay for Discoloration of Reactive Red 4 Dye from Aqueous Solution
Amirhossein Monzavi1, Majid Montazer2 and Reza Mohammad Ali Malek3
1Department of Textile Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran.
2Department of Textile Engineering, Functional Fibrous Structures & Environmental Enhancement (FFSEE), Amirkabir University of Technology, Tehran, Iran.
3Department of Textile Engineering, Center of Excellence in Textile, Amirkabir University of Technology, Tehran, Iran.
Corresponding Author E-mail: tex5mm@aut.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/330450
Here, a commercially available absorbent with a high adsorption capacity (Cloisite® 15A) was coated on a relatively cheap and flexible polyester fabric through simple pad–dry–cure method for removing dye from an aqueous solution. By using this component with good activity against discoloration expected to produce a substrate with a good ability discoloration. FESEM images with EDX patterns and X–ray diffraction spectra revealed the presence of nanoclay on the fabric surface. Discoloration of Reactive Red 4 dye (RR4) was performed by addition of a piece of the coated fabric with stirring in the dye solution. The surface of the coated fabric was colored by the presence of nanoclay acts as adsorbent dye.
KEYWORDS:Discoloration; Reactive Red 4 dye; Nanoclay; Polyester fabric
Download this article as:| Copy the following to cite this article: Monzavi A, Montazer M, Malek R. M. A. A Novel Polyester Fabric Coated with Nanoclay for Discoloration of Reactive Red 4 Dye from Aqueous Solution. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Monzavi A, Montazer M, Malek R. M. A. A Novel Polyester Fabric Coated with Nanoclay for Discoloration of Reactive Red 4 Dye from Aqueous Solution. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=34349 |
Introduction
Reactive dyes are the most important dye for the dyeing of different textile substrates, in particular cellulosic fibers.1,2. The wash fastness properties of dyed fabric with reactive dyes are classified as very good3,4. Textile dyes are one of the main pollutants in textile effluents by reducing light penetration and dissolved oxygen inhibition aquatic photosynthesis5. Even the inefficient fixation and hydrolysis in water leaves large amounts of non–fixed dye and hydrolyzed as effluents6,7. The presence of very small amount of the dye is visible in the effluent. Therefore, discoloration of reactive dyes containing textile wastewater before discharge is necessary. The reactive dyes are stable to heat, light and oxidants8,10.
The conventional effluent treatments are ineffective for the removal of dye due to the toxicity and degradation resistant of reactive dyes11,12. Some physical and chemical techniques have been reported for the removal of reactive dyes as ozonation, activated carbon adsorption, biodegradation, and advanced oxidation processes13,16. Recently, they have been used advanced oxidation processes with ozone and the ozone–based method as a technology capable of degradation of organic pollutants17,19.
Adsorption techniques have also attracted attention in recent years because of their effectiveness in the removal of polluting effluent. Montmorillonite clay typically is to form a very soft phyllosilicate mineral formed in a microscopic crystal.This is made plate–shaped mineral particles with an average diameter of one micron. It is an important component of weathering volcanic ash, bentonite, with the particles of extremely small thickness (~ 1 nm)20,24.
Yang et al., used nanoclay, montmorillonite, and some nanoclay modified as sorbents for non–ionic, anionic and cationic dyes. They have demonstrated that with some modifications on nanoclay, an excellent sorbent can be obtained for textile dyes25. Liu et al., activated raw clays by acid treatment or calcinations and organic–modified clays with small molecules or polymers to adsorb and remove organic dyes from aqueous solutions26.
Woven fabric made of polyester (PET) is cheap and flexible, with high mechanical strength and surface area. The construction of the flexible fabric allows the rapid removal of the dye in the solution. In addition, the open design ensures optimal surface and substrate turnover active easily changed from the fiber diameter27,28. In this study, nanoclay coat was applied to the PET fabric by pad–dry–cure technique and the structure was demonstrated by FTIR, FESEM and XRD. The reactive dye adsorption on samples described studying the influence of various parameters, including the contact time and adsorbent dosage of the solution. Moreover, the fabric on both sides can be used for the adsorption.
Materials and Methods
Cibacron Brilliant Red 3B–A (Reactive Red 4) was supplied by Sigma–Aldrich Chemie GmbH (Germany) with empirical formula C32H24ClN8Na4O14S4, molar weight of 1,000.25 g/mol and λmax=517 nm. The chemical structure of the dye is shown in Fig. 1(A). Modified montmorillonite with a quaternary ammonium salt (Cloisite® 15A) was obtained from Southern Clay Products, Inc (USA). The chemical and physical structure of the nanoclay is shown in Fig. 1(B). A 100 % polyester woven fabric weighing 97 g ⁄m2 with a yarn count of 150 Den and wrap/weft density of 30/22 yarn/cm was purchased from HEJAB TEXTILE CO. (Tehran, Iran).
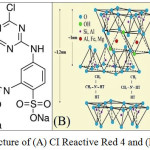 |
Figure 1: The chemical structure of (A) CI Reactive Red 4 and (B) Cloisite® 15A. |
Preparation and Coat Nanomaterials on the Fabric
The aqueous dispersion was prepared by mixing 3–20 (w/v %) nanoclay and distilled water needed in a bath with a liquor goods ratio of 40:1 for 15 minutes at 30–40 °C. Non–ionic detergent was used for the washing of polyester fabric before treatment. The washed fabric was impregnated in the dispersion padded with 90 % wet pick–up dried at 100 °C for 4 minutes followed by curing at 190 °C for 7 minutes in a thermal oven (Binder, Germany).
Procedure
First, 0.25g coated fabric was cut and placed in the beaker. 25mL dye solution with the concentration of 20ppm was added to the beaker and exposed to stirring for a few minutes. The absorbance spectra of the solution finally registered. The experimental set–up was based on absorbance reaction apparatus made of glass with dimensions of 3x3cm. dye solution, including fabric coated with nanomaterials poured from the to pof the container. The container was washed after each run of the experiment and a new piece of coated fabric was used later.
Analytical Procedures
The aqueous solution absorbance was monitored with a double beam UV/VIS spectrophotometer (Uvikon 923, Bio–Tek Kontron Instruments). Fourier Transform Infrared (FTIR) spectra were registered using the Thermo Nicolet. The microscopic images were obtained from a Field Emission Scanning Electron Microscope (FESEM; MIRA3 XMU, Tescan). Energy dispersive X–ray spectroscopy (EDS) point analysis and mapping with the Oxford AZTEC EBSD–EDS system was performed. X–ray diffraction analysis from the Seifert XRD 3003 PTS diffractometer system was used, with the Rayflex software. Different fabric samples prepared according to Table 1.
Table 1: Fabric preparation for discoloration, including nanaoclay 15A
|
Fabric No. |
1 |
2 |
3 |
4 |
5 |
|
15A (w/v %) |
3.00 |
6.45 |
11.50 |
16.55 |
20.00 |
Nanoclays modified with a quaternary ammonium salt is used as a strong adsorbing dye. However, if the layers of nanoclay exfoliation, the adsorption capacity increases dramatically. With the opening of the clay layers by stirring in the treatment bath (Fig. 2), the dye molecules adsorbed to the layers. Witch stirring nanoclays are well dispersed in an aqueous medium. Through the exfoliation of the nanoclay layers, the ammonium salts are situated between the plates.
Result and Discussion
After impregnation of the fabric in the dispersion solution, the fabric was padded with the indicated wet pickup, dried and cured to fix the nanomaterials on the substrate. The distribution of the nanoparticles on the fabric substrate is shown in Fig.2.
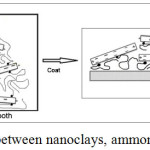 |
Figure 2: Schematic of interaction between nanoclays, ammonium salts and polyester fabric.
|
FTIR Analysis
The FTIR spectra of the polyester fabric before and after coat with nanoclay are illustrated in Fig. 3. The saturated esters related peaks were found at 1729 cm–1 due to carbonyl (─C═O) stretching. The bands around 1330–1240 and 1160–1080 cm–1 is due to vibration of the ester group [29]. Also the ─C─H bonds linked with the benzene ring in the polymer structure show shades peaks around 1600–1400 cm–1 with beyond the plane of the benzene ring at 1018, 875 and 730 cm–1 [30]. The characteristic bands of nanoclay can be observed prior to 1100 cm–1. A peak at 1020 cm–1 belongs to the stretching vibration of Si─O bonds of the tetrahedral silica layers. The peaks at 719, 619, 519 and 451 cm–1 correspond to Si─O and Al─O bending vibration of the octahedral and tetrahedral layers of silica–alumina. The bands have appeared at 2845 and 2920 cm–1 are specific to stretching vibration of ─CH2 and ─CH3 of alkyl ammonium cation, respectively [31]. The ─CH bending vibration of ─CH3 is also observed at 1465 cm–1. The stretching vibration of ─OH groups are characterized by a band at 3622 cm–1 [32].
FESEM Images
FESEM images of various polyester fabrics including untreated and treated with nanoclay are presented in Fig. 3. This is used to confirm the load nanoparticles on the surface of the substrate for the additional dye removal. Fig. 3(a) showed the surface of the polyester fiber (control sample) with a clean surface and not loaded nanoparticles. According to Fig. 3(b) the nanoclay coated sample compared to the uncoated sample which nanoclay loaded on the fabric surface. To estimate the average thickness of nanoclay layer distributed on the fabric surface, SEM images were taken at 75.0 KX. It is revealed that the nanoclay plates with a thickness of about 10 nm are uniformly distributed on the fabric surface. SEM image of 3(b) has confirmed that the surface characteristics of the fabrics modified after loading and one layer of nanoclay extensively covered, which implies an even loading of nanomaterials. The average thickness of nanoclay layer observed about 10–11 nm.
EDS Pattern
The presence of nanomaterials on the fabrics structure was investigated by energy dispersive X–ray spectroscopy. ED S spectrum of polyester fabric sample treated with the nanoclay was examined to understand the basic specification of the fabric (Fig.3). Photo electron peaks were observed at about 200 eV, it shows the presence of Si ,Al and Mg, which is an other proof of nanoclay particles on the fabric surface and the presence of C and O is due to the polyester chains. The presence of nanoclay on the surface of the treated fabric has been confirmed that the sign of Si, Al and Mg peaks indicated on rough fabric. Au peaks areal so included in the spectrum as the processed sample was coated with gold before EDS study.
X–Ray Diffraction
The XRD patterns of different samples, including control and treated fabric illustrated in Fig. 3. The polyester characteristic peaks in the spectra are identified with the star at Bragg angles of 17.62, 23.11 and 25.57° [33]. Two new peaks at 5.04 and 6.69° denoted the presence of nanoclay on the fabric surface [34]. It can be seen that d (001) than the raw clay presented to 7.64°, which corresponds to the layer spacing of 1.15 nm. The method to be of clay on the fabric surface is determined according to the evolution and the intensity peak. The appearance of new peaks at 5.49 and 5.72° in the treated sample with nanoclay d=1.6 and 1.7 nm represented the increasing distance between the corresponding clay layers. Therefore, the presence of the quaternary ammonium salt has led to the intercalation between the clay sheets. Other reflections have appeared in the XRD pattern attributed to disorientation hydrogenated tallow alkyl between the clay sheets. Despite these diffraction peaks can potentially produce more nanoclay content on the fabric, which led the aggregations, however, the degree of aggregation were quite low due to the low intensity of diffraction peaks at 4.64°. The other peaks decreased slightly and shifted due to delaminating and confusion of clay sheets [35].
 |
Figure 3: FESEM images, FTIR spectra, XRD and EDS patterns of various polyester fabrics (a) untreated and (b) coated with nanoclay. |
The large surface area to mass ratio of nanomaterials significantly improves the adsorption capacity as the absorbent. In addition to high specific surfaces, nanoparticles have exclusive adsorption due to different distributions of reactive surface sites and disturbed surfaces. Adsorption of organics to the nanoclay media has been extremely rapid.
The most important absorbance peak in the visible spectrum of Cibacron Brilliant Red 3B–A can be specified at 517 nm and can be used to measure the discoloration of the dye solution. The solution adsorption values for different conditions of stirring time are shown in Fig. 4. The dye concentration in the solution indicates that the adsorption equilibrium of the dye to the treated fabric is greater than that of the untreated one. On this basis, nanoclay increases adsorption of the dye on the fabric and acts as the most important criteria in the discoloration.
The changes in the UV–visible spectrum of reactive dye solutions were monitored to investigate the mechanism responsible for the discoloration. The results are shown in Fig. 4. Adsorption of reactive dyes of clay nanosheets is due to chemical interaction between the dyes and distribution of the various functional units in the interlayer spacing of two dimensional layered nanoclays.
The adsorption of dye solutions depends on many factors including the type, pH and the concentration of the dye. At high concentrations of reactive dyes used in the present study (20 ppm), the adsorption capacity may be low.
![Figure 4: Absorbance spectra of dye solutions [CI Reactive Red 4 (20 ppm)] discolored using polyester fabric treated with (a) nanoclay and the control sample (b) under stirring.](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_nove_Ami_fig4-150x150.jpg) |
Figure 4: Absorbance spectra of dye solutions [CI Reactive Red 4 (20 ppm)] discolored using polyester fabric treated with (a) nanoclay and the control sample (b) under stirring. Click here to View figure |
The results in Fig. 4 also show that the adsorption of dye increases with an increase of the stirring time. The appearance of red color on polyester fabric coated with the dispersion of nanoclay is a visible proof of the greater ability of dye sorption. The chemisorptions of the anionic reactive dyes on polyester treated with nanoclay mainly due to the electrostatic attraction between the nanoclay ammonium salt groups and fully deprotonated sulfonic groups of the dye solutions [36]. During the periods of discoloration, the peak of the maximum wavelength was stable. Thus, by coating the polyester substrate with nanoclay sheets, adsorption initially played a dominant role for dye removal. As the discoloration was completed at the end of the shaking, the absorbance peak for all the procedures is roughly disappeared, and a strong red dye solution became colorless.
Conclusion
The textile materials have been successfully introduced as an excellent substrate for the immobilization of nanomaterials on the surface to remove the dye from dyeing effluents. Adsorption of Reactive Red 4 from the aqueous solution was carried out in the presence of polyester fabric with immobilized nanoclay layers by stirring. Also the stability of nanomaterials on the fabric surface was good. The process of decolorization of the dye solution is repeated with the same substrate several times with reasonable efficiency discoloration. In this study, the objective of the dye desorption further confirms that adsorption is primarily responsible for the initial discoloration.
References
- Dolby, P.J. Textile Chemist & Colorist, 1977, 9, 32–36
- Khatri, A.; Peerzada, M. H.; Mohsin, M.; White, M. J. Clean. Prod. 2015, 87, 50–57
CrossRef - Zhang, S.; Ma, W.; Ju, B.; Dang, N.; Zhang, M.; Wu, S.; Yang, J. Color. Technol. 2005, 121, 183–186
CrossRef - Rizk, H. F.; Ibrahim, S. A.; El–Borai, M. A. Dyes Pigments, 2015, 112, 86–92
CrossRef - Correia, V. M.; Stephenson, T.; Judd, S. J. Environ. Technol. 1994, 15, 917–929
CrossRef - Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Bioresource Technol. 2001, 77, 247–255
CrossRef - Kordouli, E.; Bourikas, K.; Lycourghiotis, A.; Kordulis, C. Catal. Today, 2015, 252, 128–135
CrossRef - Renfrew, A. H. M.; Taylor, J. A. Color. Technol. 1990, 20, 1–9
CrossRef - Fujioka, S.; Abeta, S. Dyes Pigments, 1982, 3, 281–294
CrossRef - Lucic, M.; Milosavljevic, N.; Radetic, M.; Saponjic, Z.; Radoicic, M.; Krusic, M. K. Sep. Purif. Technol. 2014, 122, 206–216
CrossRef - Crini, G. Bioresource Technol. 2006, 97, 1061–1085
CrossRef - Chong, M. N.; Cho, Y. J.; Poh, P. E.; Jin, B. J. Clean. Prod. 2015, 89, 196–202
CrossRef - Ezechi, E. H.; Kutty, S. R. M.; Malakahmad, A.; Isa, M. H. Process Saf. Environ. 2015, 98, 16–32
CrossRef - Abidi, N.; Errais, E.; Duplay, J.; Berez, A.; Jrad, A.; Schafer, G.; Ghazi, M.; Semhi, K.; Trabelsi-Ayadi, M. J. Clean. Prod. 2015, 86, 432–440
CrossRef - Soboleva, N. M.; Nosovich, A. A.; Goncharuk, V. V. J. Water Chem. Technol. 2007, 29, 72–89
CrossRef - Franz, S.; Perego, D.; Marchese, O.; Bestetti, M. J. Water Chem. Technol. 2015, 37, 108–115
CrossRef - Azbar, N.; Yonar, T.; Kestioglu, K. Chemosphere, 2004, 55, 35–43
CrossRef - Radi, M. A.; Nasirizadeh, N.; Rohani–Moghadam, M.; Dehghani, M. Ultrason. Sonochem. 2015, 27, 609–615
CrossRef - Khouni, I.; Marrot, B.; Moulin, P.; Amar, R. B. Desalination, 2011, 268, 27–37
CrossRef - Elemen, S.; Kumbasar, E. P. A.; Yapar, S. Dyes Pigments, 2012, 95, 102–111
CrossRef - Zhou, C. H.; Keeling, J. Appl. Clay Sci. 2013, 74, 3–9
CrossRef - Duman, O.; Tunc, S.; Polat, T. G. Appl. Clay Sci. 2015, 109–110, 22–32
CrossRef - Ghemati, D. j.; Aliouche, D. j. J. Water Chem. Technol. 2014, 36, 265–272
CrossRef - Nadafi, K.; Vosoughi, M.; Asadi, A.; Omidvar Borna, M.; Shirmardi, M. J. Water Chem. Technol. 2014, 36, 125–133
CrossRef - Yang, Y.; Han, S.; Fan, Q.; Ugbolue, S. C. Text. Res. J. 2005, 75, 622–627
CrossRef - Liu, P.; Zhang, L. Sep. Purif. Technol. 2007, 58, 32–39
CrossRef - Radetic, M. J. Photoch. Photobio. C. 2013, 16, 62–76
- Farouk, A.; Sharaf, S.; El–Hady, M. M. A. Int. J. Biol. Macromol. 2013, 61, 230–237
CrossRef - Cole, K. C.; Daly, H. B.; Sanschagrin, B.; Nguyen, K. T.; Ajji, A. Polymer, 1999, 40, 3505–3513
CrossRef - Mejia, M. I.; Marin, J. M.; Restrepo, G.; Rios, L. A.; Pulgarin, C.; Kiwi, J. Appl. Catal. B-Environ. 2010, 94, 166–172
- Dalir, H.; Farahani, R. D.; Nhim, V.; Samson, B.; Levesque, M.; Therriault, D. Langmuir, 2012, 28, 791–803
CrossRef - Kozak, M.; Domka, L. J. Phys. Chem. Solids, 2004, 65, 441–445
CrossRef - Chang, J. H.; Kim, S. J.; Joo, Y. L.; Im, S. Polymer, 2004, 45, 919–926
CrossRef - Phang, I. Y.; Pramoda, K. P.; Liu, T.; He, C. Polym. Int. 2004, 53, 1282–1289
CrossRef - Asadi, M.; Montazer, M. J. Inorg. Organomet. P. 2013, 23, 1358–1367
CrossRef - Wangpradit, R.; Chitprasert, P. Int. Biodeter. Biodegr. 2014, 93, 168–176
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









