Synthesis of Mixed Metal Oxides Nano-Colloidal Particles and Investigation of the Cytotoxicity Effects on the Human Pulmonary Cell Lines: A Prospective Approach in Anti-Tuberculosis Inhaled Nanoparticles
Alireza Jafari1, Sharmin Kharazi2, Nader Mosavari3, Farahnaz Movahedzadeh4,5, Majid Tebyaniyan6, Saeedeh Jafari Nodooshan7, Ali Majidpour1,8 and Tahereh Mosavi1
1Antimicrobial Resistance Research Center, Rasoul-e-Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
2Department of Medical Nanotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3Reference Laboratory for Bovine Tuberculosis, Razi Vaccine and Serum Research Institute, Agricultural Research Education and Extension Organization, Tehran, Iran.
4Institute for Tuberculosis Research, College of Pharmacy, University of Illinois at Chicago, Chicago, Illinois, United States of America.
5Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy University of Illinois at Chicago, Chicago, Illinois, United States of America.
6Razi Vaccine and Serum Research Institute, Agricultural Research Education and Extension Organization (AREEO), Tehran, Iran.
7Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
8Department of Infection Disease, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
Corresponding Author E-mail: Taherehtavakol@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330358
In today's world, Nano-science in association with synthesis of effective anti-tuberculosis nanoparticles (NPs) is expanding day by day. Also, personalized medicine in the field of Mycobacteriology; will play a significant role in the diagnosis, control, and even treatment of infectious diseases, especially tuberculosis in the next future. In this study, the colloidal Ag & ZnO NPs were synthesized with chemical reduction and deposition method. The SEM, TEM, DLS, UV-visible, and ICP-MS were used to identify of characteristics, structure, and estimate of NPS size, as well as the dispersion. Next, NPs were mixed together with 2Ag:8ZnO, 5Ag:5ZnO and 8Ag:2ZnO ratios. The toxicity effects of different ratios of NPs in contrast to human macrophage (THP-1) and normal human lung fibroblast (MRC-5) cell lines were evaluated using MTT method. The average size of NPs were estimated about 13±3.14 nm and 4±0.88 nm, respectively. The NPs were spherical in shape with a smooth surface morphology, as well as agglomerates. The results of UV- visible spectrums shows two peak in 420 nm and 350 nm for NPs, respectively. The intensity distribution spectrum indicate that mid-range of hydrodynamic diameter of the NPs were about 28.91 nm and 57 nm. The PDI and zeta potential of NPs were 1.00 and 0.545, also -20.7 mV and 3.13 mV, respectively. The results showed that the 5Ag:5ZnO ratio of mixed NPs in the dilution of 1:64 0.663 ppm), did not have any toxicity effects against THP-1 and MRC-5 cells. The ZnO NPs have had strongly toxic effects on THP-1 and MRC-5. Also, in the dilution of 1:64 of Ag NPs ( 0.390 ppm), we did not find any evidence case of toxicity against normal lung and THP-1 cell lines. We are looking for exclusive ratio : dilution of NPs that used in the next future as a safety anti-tuberculosis agent in the personalized medicine and treatment process of respiratory disease; spatially “tuberculosis”. We discovered, if the NPs are mixed with a ratio of 5:5 in dilution of 1:64 0.663 ppm) of initial concentration, we will not see any toxic effects on the human respiratory cells.
KEYWORDS:Mixed Nano-colloidal metal oxides; Cytotoxicity; THP-1; MRC-5; Anti-tuberculosis; Personalized medicine
Download this article as:| Copy the following to cite this article: Jafari A, Kharazi S, Mosavari N, Movahedzadeh F, Tebyaniyan M, Nodooshan S. J, Majidpour A, Mosavi T. Synthesis of Mixed Metal Oxides Nano-Colloidal Particles and Investigation of the Cytotoxicity Effects on the Human Pulmonary Cell Lines: A Prospective Approach in Anti-Tuberculosis Inhaled Nanoparticles. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Jafari A, Kharazi S, Mosavari N, Movahedzadeh F, Tebyaniyan M, Nodooshan S. J, Majidpour A, Mosavi T. Synthesis of Mixed Metal Oxides Nano-Colloidal Particles and Investigation of the Cytotoxicity Effects on the Human Pulmonary Cell Lines: A Prospective Approach in Anti-Tuberculosis Inhaled Nanoparticles. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33977 |
Introduction
In recent years, nanotechnology has made enormous progress in the fields of biology and medical science1. Metal oxide nanoparticles (NPs), compared with other materials exhibit different properties. Many properties have dependent to the NPs size, physical characteristics, surface area, magnetic, mechanical, optical as well as chemical properties such as reactivity and thermal properties. Because of these unique properties, today the NPs are used to treat infectious diseases, cancer biology and medical science research1. From ancient times, Ag and ZnO have been used as an antibacterial. So that in ancient Greece, Rome, and Macedonia, metal oxide NPs were used to control infections. But in recent years, research has focused on the novel anti-tuberculosis agents and also the role of nanotechnology and personalized medicine in the diagnosis, treatment and elimination of tuberculosis from human society. However, since the discovery of this NPs, the scientists have been concerned about the environmental impact and human exposure. Hazard and risk assessment of metal oxide NPs mainly to be assessed through the mobility, large surface area, the permeability and the absorbency to the cell1. As metal oxide NPs such as Ag and ZnO NPs which can be absorbed by plants or other living organisms and reach the food chain1.It was not worthy that until ten years ago, all scientists believed that Ag and ZnO NPs are non-toxic to mammalian cells2. The main way of uptake of NPs were into the human body via the skin, the respiratory tract, or the gastrointestinal tract1. Thereby, NPs size, shape, and surface modification play an important role in distribution in the organism. The absorbed NPs via the respiratory tract can reach the lymph stream and the blood circulation3. Some studies showed that the NPs are able to pass through the blood-brain-barrier4 and the cell membranes5-6and can thus deposit in organs and interact with biological systems. It has been shown that Ag NPs can induce a toxic response of different mammalian cell lines7-8. Hence, Ag NPs exposure resulted in a decreased viability or the release of lactate dehydrogenase in rat liver cells9, in mouse germline stem cells, in human fibroblasts, and in rat adrenal cells10.
Tuberculosis (TB) is a chronic and contagious bacterial infection that caused by various strains of Mycobacterium, especially M. tuberculosis. TB is mainly confined to the lungs but can also expand as extra-pulmonary TB in various parts of the body including eye, bone, neurons or blood vessels. Currently, one third of the world’s population is tainted with TB, and if someone with active TB is left untreated then on average between 10 and 15 people can be infected every year11. In a global report, the ,WHO estimated 9.4 million TB cases in 2008 out of which 1.98 million cases were observed from India alone. Thus, India accounts for one fifth of the global burden of TB case reports. The clinical cure of TB is turning out to be a difficult task because of emergence of multidrug-resistant (MDR) tuberculin bacterial strains (MDR-TB). Current estimation indicates that out of all incidents of TB cases, about 3.6% are reported to have MDR-TB12. Studies have shown that ZnO NPs induce the production of the pro-inflammatory cytokines, IFN-γ, TNF-α, and IL-12, at concentrations below those causing appreciable cell death. In fact, T and B lymphocytes had more resistant to ZnO NPs toxicity compared to monocytes and NK cells. They also discovered that ZnO NPs induce ROS production in monocytes and T cells. Also, ZnO NPs preferentially associate with monocytes compared to lymphocytes13. In the TB, Mycobacterium tuberculosis enters the respiratory tract and small infectious particles penetrate into the lung alveolar, where that was phagocyte by alveolar macrophages. Mycobacterium tuberculosis prevents phagosome and lysosome integration by blocking EEA1. Phagocytic bacteria are able to escape from active nitrogen intermediates by catabolizing oxidizing agents. Following up the induced response of bacteria, chemotactic factors (complement component C5a), macrophage and circulation lymphocyte are also attracted to the infection center. As a result of this, process will form giant cells and granuloma. Subsequently, Intracellular growth of mycobacteria in macrophage stimulated CD4+ and CD8+cells. This activation of CD4+ cells led to stimulate to produce antibody but that is no effective. Also, release of T cells of Interferon gamma (IFNγ) and other cytokines can be activated macrophage. The localization of activated macrophages interdict the extension of bacteria. It means that they can penetrate to the granuloma and kill all organisms in it. However, the necrotic granulomatous was covered with fibrin and so Mtb can be continued hidden life. The remarkable point is that, it could be reactive when the host immune response is weak14.
Personalized medicine is currently one of the driving forces for new research and discoveries. In the next five years, personalized medicine is expected to be the main goal in biomedical research15. Although, the personalized medicine is new but a rapidly advancing field of healthcare. Personalized medicine is about making the treatment as individualized as the disease. It involves identifying genetic, genomic, proteomic and clinical information in order to make accurate predictions about a person’s susceptibility to developing disease, the course of disease, and its response to treatment. Finding personalized biomarkers to best define chemotherapy protocols, individualized treatment duration, and predict risk of adverse events with antibiotics would be other interesting markers for clinicians16.
The aim of this study was synthesis of mixed ratios of 2Ag:8ZnO, 5Ag:5ZnO, and 8Ag:2ZnO heterogeneous metal oxides nano-colloidal particles and investigation of the cytotoxicity effects of each of the ratios: dilutions on the human macrophage and normal lung fibroblast cell lines; these 2 types of cell were chosen because they are potential targets of nanomaterials in vivo after inhalation17.So that we can find a safe and suitable rang of the ratios: dilutions of NPs, that both THP-1 and MRC-5 cells are still alive. So maybe by doing this study, we can imagine the bright future for safety anti-tuberculosis agents and inhibitory of Mtb growth by nanotechnology and personalized medicine.
Materials and Methods
The Synthesis of Colloidal Ag Nps
At first,0.5 mM silver nitrate (AgNO3) (MERCK, Germany) is prepared to volume 12ml. In the next step, 0.02 M sodium citrate (Na3C6H5O7)(MERCK, Germany) is prepared to volume 0.5 ml. Sodium citrate is added to the silver nitrate solution. Sodium citrate solution was used to stabilize the silver NPs. Finally, 0.5 ml Sodium borohydride (NaBH4) (MERCK, Germany) 0.01M is prepared at room temperature. Since the Sodium borohydride is an active compound distilled water was used to prepare it. The Sodium borohydride compound is placed at -18 ° C for 10 min. For the synthesis of silver NPs, sodium borohydride was added to sodium citrate and silver nitrate, as quickly as possible. The final product for 10 min at room temperature, was stirred on a magnetic stirrer18.
The Synthesis of Colloidal Zno Nps
To prepare 0.2 M of zinc acetate (C4H6O4Zn ) (Merck, Germany), 1.0974 g of zinc acetate is added to 25 ml of ethanol (C2H6O) (Merck, Germany). Then a solution of zinc acetate was placed on a magnetic stirrer at room temperature to obtain a clear solution. In another flask, 1.2 gr of Sodium hydroxide (NaOH) (Merck, Germany) was added in 25ml of methanol, then to obtain a clear solution, it is placed on a magnetic stirrer at room temperature. Then, the zinc acetate solution (1.2 M) was added drop by drop to a solution of sodium hydroxide. Dropping time should be uniform period. The final solution should be placed on a magnetic stirrer for three hours to complete the synthesis of zinc oxide NPs. Finally, milky solution containing zinc oxide NPs obtained. The synthesized solution was centrifuged for the separation of zinc oxide NPs. At each stage, to disperse the particles, it is placed in an ultrasonic bath until these thoroughly dispersed in a solvent. Next, the ZnO nano-colloidal particle was poured on clean glass plate to evaporate the ethanol at room temperature. Dried material in a glass plate was milled by mechanical methods. The nano-powder was mixed with 50 ml of deionized distilled water and then was sonicated19.
The UV-Visible Spectrophotometry
The absorption spectrums of Ag NPs and ZnO NPs were done by Scan Drop UV-visible spectrophotometer (Analyticgena, Germany). Separately, about 2 ml of Ag and ZnO NPs, with a ratio of 1:1 was diluted with deionized distilled water. Then were poured into the cells. Next, wavelength of absorption of NPs was scanned from 200nm to 800 nm20-21.
The Dynamic Light Scattering (DLS), Inter-Quartile Coefficient of Skewness (IQCS), the Poly Dispersity Index (PDI), Zeta Potential
The Dynamic light scattering (DLS) measurements to determine the middle size, and zeta potential or electrical potential in colloidal state of Ag & ZnO NPs were performed using a Scatteroscope-I (K-ONE Inc., Korea).Thus,1 ml of NPs suspensions were mixed with 1 ml distilled water, sonicated in a nice bath for 30s, and added to the sample dispersion unit for size analysis. Middle hydrodynamic particles size was calculated using of obtained information. Inter-Quartile Coefficient of Skewness of Ag and ZnO NPs was calculated by down formula22-23.
![]()
If the value of the formula is zero, the system will be fully mono-disperse. While the IQCS indicates values of -1 or +1, the system will be the maximum amount of poly-disperse. The wide distribution was indicated by the poly dispersity index (PDI)24.
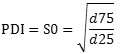
In this formula, if the value of S0 is closer to number one, all of nanoparticles will be mono-dispersed. And so, if S is larger than number 1, in this case NPs are made of dispersants. Zeta potential was measured by the same instrument following the same protocol. All measurements were performed in triplicates at 25oC.
Inductively Coupled Plasma Mass Spectrometry
ICP-MS has become the technique of choice for detection and characterization of NPs in solution. Compared with other techniques, ICP-MS is unique in its ability to provide information on NPs size, size distribution, elemental composition, and number concentration in a single, rapid analysis. In addition, only ICP-MS can simultaneously determine the concentration of dissolved analyze in the sample. The prepared samples were analyzed by ICP-MS (NexION® 350Q, Perkin Elmer, USA)25-26.
The Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
The morphology of Ag & ZnO NPs were analyzed using scanning electron microscopy (SEM) (Hitachi, S4169 Japon). The NPs suspensions were diluted in distilled water and dropped onto copper stubs. After air drying, particles were coated with a thin layer of gold under vacuum to make them conductive. Surface was scanned and photographs were taken at a 10 kV acceleratingvoltage27-28. The Transmission electron microscopy(TEM)is one of the highest resolution analysis methods of materials. The three dimensional recognition of the materials is difficult by TEM because the observation data is a projection images through the materials29. For morphological assessment, one drop was placed on a carbon coated copper grid and the excess amount was removed with a piece of filter paper. A drop of aqueous solution of Phosphortungistic acid (H3PW12O40) (MERCK, Germany) was added onto the grid and staining was allowed for 5 min at room temperature. The prepared sample was then evaluated by TEM using a CM 120 (Philips, Germany) instrument at 200KV30.
The Preparing of Different Ratios of Colloidal Ag & ZnO NPs
To prepare the mixture of 2Ag:8ZnO, 5Ag:5ZnOand 8Ag:2ZnO NPs, Ag & ZnO NPs, dispersed in a cold ultrasonic bath to 25oC for 30s,before used. For the preparation of 10 ml of8ZnO: 2AgNPs ratios, 2 ml of Ag NPs with a value of 8 ml of ZnO NPs were mixed in a sterile Falcon tube. Also, to prepare 10 ml mixture 5ZnO:5Ag of Ag & ZnO NPs, 5 ml of Ag NPs with 5 ml of ZnO NPs were mixed. The 2ZnO: 8Agratios of NPs was performed also according to the table I. Also, The approximate concentration of Ag NPs, ZnO NPs and 8ZnO:2Ag, 5ZnO:5Ag and 2ZnO:8Ag mixed NPs in different dilution in a final volume of 1 ml calculated in table II.
Table 1: The approximate concentration of Ag NPs, ZnO NPs and 8ZnO:2Ag, 5ZnO:5Ag and 2ZnO:8Ag mixed NPs in a final volume of 10 ml.
|
Nanoparticle |
Ag |
ZnO |
Ag:ZnO |
|
Ag |
200 ppm per 10 ml |
0 |
– |
|
ZnO |
0 |
600ppm per 10 ml |
– |
|
2Ag:8ZnO |
40 ppm per 2 ml |
480 ppm per 8 ml |
40Ag:480ZnO |
|
5Ag:5ZnO |
100 ppm per 5 ml |
300 ppm per 5 ml |
100 Ag:300ZnO |
|
8Ag:2ZnO |
120 ppm per 2 ml |
160 ppm per 8 ml |
120 Ag:160ZnO |
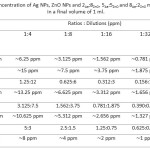 |
Table 2: The approximate concentration of Ag NPs, ZnO NPs and 2Ag:8ZnO, 5Ag:5ZnO and 8Ag:2ZnO mixed NPs in different dilution in a final volume of 1 ml. |
The Preparation of Different Ratios: Dilution of Mixed Nps in Cell Culture Medium
Firstly, about 11 Falcon tubes were prepared containing 10 ml sterile Gibco RPMI 1640 medium for serial dilution of the NPs in cell culture medium. Subsequently, all of the falcon tubes were numbered and was floated in cold ultrasonic baths at room temperature for 30s. Next, 10 ml (about 200 ppm) of the Ag NPs was added to the tube (number 1). At this time, the concentration of silver NPs in first falcon tubes (1) was reduced to the half of initial concentration. After Pipetting, about 10 ml of number 1 falcon tube was added to the second falcon tube (number 2). Continuously, serial dilution of NPs was continued until the last falcon tube (number 9) and also, variety of obtained concentrations for each dilution was approximately calculated. Separately, the serial dilution of the ZnO NPs, 2Ag:8ZnO, 5Ag:5ZnO, and 8Ag:2ZnO of mixed NPs were performed. The last two Falcon tubes (number 10 & 11) did not have any NPs. They were containing only RPMI and were selected as controls. Also, Dilution for different ratios of nanoparticles in a medium (DMEM-F12) medium (Sigma-Aldrich) for MRC-5 cell line was carried out with the same method.
The THP-1 and MRC-5 Cell Cultures
The THP-1and MRC-5 cell lines that had been obtained from cell bank of the Pasteur Institute of Iran (IPI). The THP-1had been derived from the peripheral blood of a 1-year-old male with acute monocyte leukemia. These cells also were phagocytic. Also, the MRC-5 cell line had been derived from normal lung tissue of a 14-week-old male fetus. The THP-1 cells were seeded in GibcoRPMI-1640 medium with Gibco FBS 10% and the penicillin 100 UNIT.ml-1 (Sigma-Aldrich) and the streptomycin 100 mg.ml-1 (Sigma-Aldrich), at 37°C and CO2 5%. When the cell density reached to 80%, about 104cell/well of these cells were seeded in 96-well plates. In addition, the MRC-5 cells were seeded in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM-F12) medium (Sigma-Aldrich) and Gibco FBS 10%.
MTT Assay
The effect of Ag NPs, ZnO NPs and 8ZnO:2Ag, 5ZnO:5Ag and 2ZnO:8Agofmixed NPs on the viability of a Human Monocyte (THP-1) and Normal Lung (MRC-5) cell lines in 24h were determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. The cells (104cell/well) were seeded in GibcoRPMI-1640 medium(200 ml per well) in 96-well plates and incubated at 37 oC in 5% CO2for 24 h, to induce cell adherence. Next, dilution of 1:2, 1:4, 1:8, 1:16, 1:32 and 1:64 of each of the NPs were prepared. Separately, the cells were treated with 100 ml of freshly prepared NPs (Ag, ZnO and 2Ag:8ZnO, 5Ag:5ZnO, and 8Ag:2ZnO mixed NPs) and further incubated at 37 oC in 5% CO2 for 48 h. For the MTT assay, 20ml of MTT solution (5 mg.mL-1 in incomplete medium) was added and incubated for 4–6 h. Next, 100 ml DMSO was added and the plates put on a shaker for 5 minutes. Absorbance was measured at 560 nm using a microtiter plate reader (Biotech, USA). The absorbance of the test group (treated cells) and control group (untreated cells) was used for the determination of cell viability percentage31.Cell survival in control cells was assumed to be100%. The percentage of cells viability were calculated by using the following equation:
![]()
Statistical Analysis
Statistical analyses were done using non-parametric form of variance, kruskalwall is tests and non-parametric form of t-test, Mann whitney u test (u-test) as appropriate. Longitudinal comparisons between the groups during the intervention were performed. Results were expressed as mean ± standard deviation of the mean (SD). The statistical significance threshold was defined as P-value≤ 0.05.The amount of the IC50 and EC50 was estimated through Graph pad prisim (version 6).All of charts were done by Microsoft office Excel software (Version 2010).
Results
Characterization of NPs
The UV-Visible Spectroscopic
In order to prove the existence of Ag & ZnO NPs, UV-visible spectrum was prepared. One of the interesting characteristics of metal NPs is their optical properties, which varies according to the size and shape of NPs. Surface Plasmon resonance of metallic NPs is responsible for the unique optical properties of them. These characteristics will vary with the size, shape, spacing from each other and the refractive index of the surrounding NPs32. Figure I shows that absorption of Ag & ZnO NPs were between 200 nm and 800 nm. Characteristic absorption of surface Plasmon resonance band was occurred at 420 nm and 350 nm, respectively.
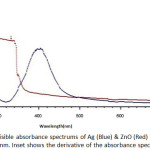 |
Figure 1: The UV-visible absorbance spectrums of Ag (Blue) & ZnO (Red) NPs from 200 nm to 800 nm. Inset shows the derivative of the absorbance spectrum. |
The Dynamic Light Scattering (DLS), Polydispersity Index(PDI), Zeta Potential
The Dynamic Light Scattering (DLS) was done using Stereoscope-IN (A-ONE Enc., Korea). The results of intensity distribution spectrum indicate that mid-range of hydrodynamic diameter of the Ag & ZnO NPs colloids were about 24.4 nm and 257 nm, respectively23.
The Polydispersity Index (PDI) is a measure of the size distribution of NPs. Accordingly, the PDI of Ag & ZnO NPs were estimated about 1.00 and 0.545, respectively. Results showed that the scattering of particles was mono-dispersed. In other words, indicated that the NPs population was homogeneous. Also, Zeta potential of NPs were -20.7 mV and 3.13 mV, respectively. A high zeta potential helps the NPs to repel each other, which ensures long-term stability and inhibitsparticleaggregation31.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
The ICP-MS with different properties such as simultaneous multi-element analysis, low detection limit, and linear of the range most appropriate method for the measurement of inorganic nanoparticles such as NPs. In this test, the concentrations of 53 elements were analyzed. According to the recorded results, the amount of Ag concentration in colloidal solution was about 25 ppm and also the concentration of ZnO NPs was shown around 55 ppm33-34.
The Scanning Electron Microscopy (SEM) and Transmission electron microscopy (TEM)
The shapes and surface morphologies of these NPs were evaluated by SEM. The particles were spherical with a smooth exterior, as shown in Figure II. The use of this technique is not very accurate, there are some deficiencies. It seems that the particle size is larger than the actual size. Also, the SEM just takes photo from adhering NPs on the sample surface. Due to the extra small size of NPs on the surface, they are agglomerated. Figure II shows the SEM image of Ag & ZnO NPs. Distribution of particle size was done with a 60 K magnification on a scale of 500 nm (a) and 750 nm (b). The TEM images of the prepared Ag and ZnO NPs are shown in Figure III. The NPs were spherical in shape with a smooth surface morphology, as well as agglomerates. Also, TEM image also shows that the produced NPs are more or less uniform in size and shape34.The average size of Ag & ZnO NPs were estimated with Digimizer software (v4.1.1.0) at about 13±3.14 nm and 4±0.88 nm, respectively.
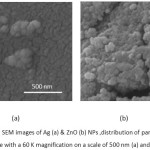 |
Figure 2: The SEM images of Ag (a) & ZnO (b) NPs ,distribution of particle size was done with a 60 K magnification on a scale of 500 nm (a) and 750 nm (b). Click here to View figure |
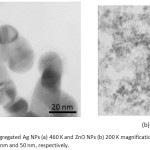 |
Figure 3: The aggregated Ag NPs (a) 460 K and ZnO NPs (b) 200 K magnification TEM images, on a scale of 20 nm and 50 nm, respectively. Click here to View figure |
MTT Assay
Cytotoxicity tests on 10 experimental groups, as well as a control group and a total of 60 tests in six different concentrations, each in three separate wells was performed(Figure IV, V and Table III, IV). Based on results mentioned, more than 50 % of THP-1 cells at a dilution of 1:8 of Ag NPs ( 3.125 ppm) in compared to the control group, were alive. When the concentration of NPs was halved, we found that cell viability increased too. So that, after dilution of 1:32 ( 0.781 ppm), about 100% of human macrophage cells were still alive. This despite the fact that, the statistical results showed that the Ag NPs at all dilutions were toxic against THP-1 cells (P-value ≤ 0.05). Interestingly, the ZnO NPs showed different behavior in front of THP-1cells and at all dilutions had toxicity effects against macrophage cells (P-value ≤ 0.05). Mixing of Ag & ZnO NPs with a ratio of 2Ag:8ZnO did not change circumstances. For this reason until dilution of 1:16 ( 3.312 ppm), less than 50% of THP-1 cells were alive. Anyway, after dilution of 1:32 ( 1.656 ppm), almost all of THP-1cells were alive. However, the P-value rang in both dilutions of 1:32 and 1:64 ( 0.828 ppm) were non-significant. This means that in these two dilutions, mixed NPs were non-toxic against macrophage cells (P-value ≥ 0.05). Mixing Ag & ZnO nanoparticles with a ratio of 2Ag:8ZnO, only at 1:32 and 1:64 dilutions showed the lowest percentage of cell viability (Up to 50%). The statistical results showed too that in 1:32 and 1:64 we have found insignificant value for P-value (P-value ≥ 0.05) that firmly say we do not have any toxicity against THP-1 cell lines. In the ratio of 5ZnO:5Ag , we had more chance. This means that at all dilutions more than 50% of cells survived. So that at dilutions of 1:16 ( 2.656 ppm), 1:32 ( 1.327 ppm), and 1:64 ( 0.663 ppm), more than 80 % of THP-1 cells compared to control group were survived (Figure IV). The statistical results showed too that at 1:32 and 1:64 dilutions of 5Ag:5ZnO we have found an insignificant value in P-value (P-value ≥ 0.05) that clearly says that we do not have any toxicity against THP-1 cell line, this could be optimistic. In other experiments, Ag NPs & ZnO NPs with the ratio of 8Ag:2ZnO were tested. In this case, the percentage of cell viability after dilution of 1:8 ( 4 ppm) increased, gradually. Besides, percentage of living cells at 1:32 (1 ppm) dilution reach to 100 %. Apparently, normal human lung cells (MRC-5) showed more sensitive to NPs (Figure V). About 70% of MRC-5 cells at 1:16 ( 1.562 ppm) dilutions of Ag NPs, were alive. This amount following of the reducing the concentration of Ag NPs is increased, gradually. Until the 1:64 ( 0.390 ppm) dilution, the percentage of cell viability compared to the control group reach to 97 %.The investigation shows that, cell viability of MRC-5 in contrast to ZnO NPs and the ratio of 8ZnO:2AgNPs were less than 50% in the all of dilutions. The statistical results showed too that in all of dilutions of 5Ag:5ZnO we have found a significant value in P-value (P-value ≥ 0.05).Undoubtedly, we can say that they have toxicity against THP-1 cell line, in all over the dilution. In addition, only at 1:64dilution of ratios 5Ag:5ZnONPs ( 0.663 ppm) and 8Ag:2ZnO NPs ( 0.5 ppm), the percentage of cell viability reach to more than 50%. Also, statistical data showed that does not exist a significant relationship between MTT results of THP-1 and MRC-5 cells after 24 hours and 48 hours. The MRC-F cells shows the most senility to the nanoparticles comparison to THP-1. So that, almost the all of the ratios: dilutions of NPs in statistic tars in spas indicated a significant value in compare to control. Apart from Ag dilutions 1:64 ( 0.390 ppm),the ratio of 5Ag:5ZnO that were significand and safe, it means that all of this ratios:dilutions are toxic against MRC-5. (P-value ≤ 0.05).
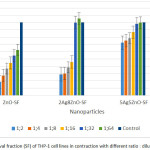 |
Figure 4: Percentage of Survival fraction (SF) of THP-1 cell lines in contraction with different ratio : dilution of Nanoparticles
|
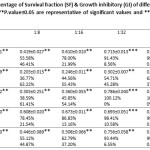 |
Table 3: The mean, standard deviation, percentage of Survival fraction (SF) & Growth inhibitory (GI) of different (ratios:dilution) of NPs in contraction with THP-1 cells after 24 hours follow-up time. (**P.value≤0.05 are representative of significant values and ***P.value≥0.05 are indicative of non-significant values). Click here to View table |
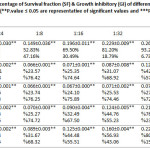 |
Table 4: The mean, standard deviation, percentage of Survival fraction (SF) & Growth inhibitory (GI) of different (ratios / dilution) of NPs in contraction with MRC-5 cells after 24 hours follow-up time. (**P.value ≤ 0.05 are representative of significant values and ***P.value ≥ 0.05 are indicative of non-significant values). Click here to View table |
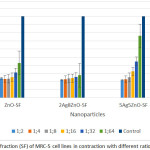 |
Figure 5: Percentage of Survival fraction (SF) of MRC-5 cell lines in contraction with different ratio / dilution of Nanoparticles Click here to View figure |
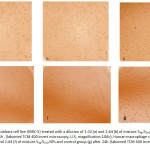 |
Figure 6: Normal lung fibroblasts cell line (MRC-5) treated with a dilution of 1:32 (a) and 1:64 (b) of mixture 5Ag:5ZnO NPs and control group (c) after 24h . (labomed TCM 400 invert microscopy, U.S, magnification 100×). Click here to View figure |
Human macrophage cell line (THP-1) treated with a dilution 1:32 (d) and 1:64 (f) of mixture 5Ag:5ZnO NPs and control group (g) after 24h. (labomed TCM 400 invert microscopy,
U.S, magnification 200×)
Table 5: The IC50 and EC50 values of Ag, ZnO, 2Ag8ZnO, 5Ag5ZnO and 8Ag2ZnO NPs in contraction with THP-1 and MRC-5 cell lines after 24 hours follow-up time. The IC50 is the concentration of an inhibitor where response (or binding is reduced by half) and The EC50 is the concentration of drug that gives half-maximal response.
|
|
THP-1 |
MRC-5 |
||
|
|
IC50 |
EC50 |
IC50 |
EC50 |
|
Ag |
˜4.212 |
˜4.490 |
˜33.14 |
˜3.394 |
|
ZnO |
˜12.13 |
˜7.340 |
˜3.194 |
≤ 1 |
|
2Ag:8ZnO |
˜5.655 ˜1.131: 4.524 |
˜5.403 ˜1.08: ˜4.322 |
≤ 1 |
≤ 1 |
|
5Ag:5ZnO |
˜1.089 ˜0.544:˜0.544 |
˜5.371 ˜2.685:˜2.685 |
≤ 1 |
≤ 1 |
|
8Ag:2ZnO |
˜3.198 ˜2.558:˜0.639 |
˜3.226 ˜2.580: ˜ 0.645 |
≤ 1 |
≤ 1 |
Discussion
At the 65th World Health Assembly in 2012, Member States, called upon WHO to develop a new post- 2015 TB strategy and targets. It is estimated that there are more than 8.7 million cases of TB in the world. More than 1.4 million of them, lose the chance to live and die. Besides, up to 0.5 million people suffer from drug resistant Mycobacterium tuberculosis (MDR) and also 150,000 of them lose their lives35. Hence, governments and organizations are always looking for drugs, novel anti-mycobacterial agents and new strategies to eliminate TB from human communities36.So it sounds as if combination of nanotechnology and personalized medicine can play a vital role in achieving this goal37-38. It was not long ago that we understand some metal oxide nanoparticles show amazing antibacterial properties against various bacteria, but there has always been concern about cytotoxicity effects of nanoparticles against human cells39-40.
In this study, we synthesized colloidal Ag & ZnO NPs by chemical deposition and chemical reduction, also compared the cytotoxicity properties against normal pulmonary cell lines at different ratios/dilutions or even individually. In fact, we have acquired the lowest concentration of ratios: dilutions nanoparticles whose they had the lowest cytotoxicity against both THP-1 and MRC-5 cell lines.
The results show that the physicochemical properties of nanoparticles were consistent with other researches. So that the UV-visible peaks for Ag & ZnO NPs were 420 nm and 350 nm, respectably41-43. UV-Visible spectroscopy is significant method which gives the preliminary confirmation of nanoparticles. Drzewiecka and their colleaqes synthesized colloidal Ag NPs in 2014 and showed the absorbance peak of Ag NPs were at 420 nm, which is characteristic feature of Ag NPs44.In addition, to determine the stability of synthesized Ag NPs, they were measured the zeta potential that was -25.2 mV.These results consistent with our zeta potential values (-20.7 mV) in this study too, which indicates that the synthesized NPs were moderately stable44.They believed that negative zeta potential value of the particles might be due to adsorption of OH– ions. Since the adsorption of OH–ions on Ag NPs increase the zeta value of NPs, there is an increase in stability of NPs due to electrostatic repulsion among the negative charges. Gurunathan also argues that an OH– ion helps in preventing the aggregate formation and maintains the smaller size of Ag NPs45. Also, the images of TEM and SEM revealed that the Ag & ZnO NPs were poly-dispersed and spherical in shape. A large number of studies give insights regarding the cytotoxicity effects excited by metal oxide NPs46.The cytotoxicity of Ag NPs has been intercommunicate with generation of reactive oxygen species. It is undeniable that several studies suggest that Ag NPs is only toxic when oxidized to silver ions, Ag+47.Asha Rani investigated the cytotoxic effect of Ag NPs on human cells. Their results illustrate that mitochondrial function as well as induction of reactive oxygen species (ROS) by Ag NPs which result in DNA damage and chromosomal aberrations48-49.The P-value in concentration of 1:32 (0.781 ppm) and 1:64 (0.390 ppm) was no significant. Thus, we can say that in these concentrations the Ag NPs do not have devastating impact on human macrophage cells. Whereas only at dilution 1:64 of Ag NPs, normal lung fibroblasts cells exhibited this behavior. The P-value of statistical data, in concentration 1:64 was more than 0.05 and showed no a significant relationship. The results demonstrated that these nanoparticles were non-toxic to MRC-5 cells. Recent studies have shown that some NP made of metal oxides, such as ZnO NPs, have selective toxicity to bacteria but exhibit minimal effect on human cells50. Even Reddy in the studies on both prokaryotic and eukaryotic cells indicated that zinc oxide nanoparticles have selective toxicity. Although, zinc oxide nanoparticles kill E. coli bacteria, are ineffective on human T lymphocytes51. Also, Sophie Lanone and their colleagues in 2009 at the University of Paris examined the cytotoxic effects of ZnO NPs in human alveolar epithelial and macrophage cell lines. The use of MTT assay on THP-1 cells indicated that the ZnO NPs is toxic52.Our research showed that zinc oxide nanoparticles have considerable toxicity to human macrophage cells and lung fibroblasts cells. So that the statistical data illustrated that, in all measured dilutions, the P-value was less than 0.05 and the toxicity seen. Generally speaking, the cell viability of THP-1 and MRC-5 cell lines, after 24 hours of incubation with ZnO NPs, measured by MTT assay designated more than 44.56% and 25.35%, survive in dilution of 1:16, respectively. This amount was amplified by decreasing of the concentration of NPs in dilution of 1:64. In other hand, it increased to 62.5% and 42.85%, respectively. In fact, the percentage of living cells, at a dilution of 1: 64, increased to 62.5% and 42.85% by reducing the concentration of nanoparticles.
According to Olesja Bondarenko researches, the reason for candidating the Ag & ZnO NPs, in this study is the metallic elemental composition of these two particles. Also, all the NPs are practical to fight the unfavorable growth of pathogenic microorganisms. Although among the two nanomaterials, Ag NPs are used most widely as antimicrobials, also ZnO NPs have been successfully used as biocides50.The next case is their negative surface charge, which results from oxygen atoms in ZnO53.They proclaimed that Ag NPs do not contain oxygen, the surface of metallic Ag NPs is oxidized under most environmental conditions (aerobic) and negatively charged hydroxo and Oxo groups cause the negative surface charge of the particle54.In terms of toxicologically, perhaps the most important share property is that all the two NPs are soluble to some extent in aqueous media. They have previously shown that the solubility of Ag & ZnO NPs is the interestingly enough issue in the toxicity of metal-containing nanoparticles55.Nonetheless, Casals and their colleagues firmly believe that solubility of NPs and the behavior of released metal ions, the proportion of intact particles, metal ions and metal complexes, depend greatly on the properties of the test environment56.It has been also emphasized that when Ag & ZnO NPs were mixed together, the antibacterial properties of NPs incredibly increased, 57-58 and represent the synergistic properties.
In current study, Salah and colleagues at 2013,researched on the inhibitory growth effects of lung fibroblasts (MRC-5) and THP-1cells in exposure on chitin, chitosan and low molecular weight chitin. The results suggest that low molecular chitin was highly suppressive. The lowest IC50 value was 1 µg cm−3 for low molecular weight chitin59.By mixing Ag & ZnO with different ratios: dilution together, their cellular cytotoxicity properties against lung cells are also changed.
Also, lung fibroblast cells compared to THP-1 cells represented more susceptible to metal oxide NPs. When Ag:ZnO NPs at a ratio of 2: 8 were mixed together, to achieve a 1:64 dilution, only 27.69% of MRC-5 cells were alive. Which was a sign of the toxicity effects of NPs on these cells. But THP-1 cells showed more differentiated behavior. In fact, dilutions of 1:32 and 1:64 of the NPs on human macrophage cells were non-toxic. But, the data which has obtained in this study, rejected the theory of using the ratio of 2:8 of Ag:ZnO NPs in body. The results of mixing of Ag:ZnO NPs at a ratio of 8:2 was also significant . Almost at dilutions of 1:32 ( 1 ppm) and 1:64 ( 0.5 ppm) More than 90% of THP-1 cells were alive. While the MRC-5 cells showed stricter behavior, in concentration of 1 ppm , about 60% of cells had survived in culture medium. As well as statistical data significantly analyzed, all the studied concentrations.
The statistical data indicates that mixed of 8: 2 NPs, is not yet an appropriate option for the treatment of pulmonary infectious diseases (P-value ≥0.05). By mixing ratio of 5Ag:5ZnONPs, less cellular cytotoxicity was also reported. So that the statistical data showed that almost 80% of lung fibroblast cells which were treated with the NPs were alive at dilution of 1:64 (P-value ≤ 0.05).
In contrast, about 100% of the human macrophage cells at dilutions of 1:32 and 1:64, were quite alive and active. (P-value ≤ 0.05). This represented, using a dilution of 1:64 to concentration of 0.663 ppm mixture of Ag:ZnO NPs at a ratio of 5:5 can be a proper option for future research on the effectiveness of non-toxic metal oxide NPs on infectious diseases which involving the lungs and respiratory system. However, Stebounova verdict that inhalation is considered the most important route of exposure for nanoparticles with bacteria 47.
Conclusion
By mixing metal nanoparticles together, can reduce the risks of cellular toxicity. By following of these studies, may imagine the treatment of pulmonary infectious diseases of the human body by Nanotechnologies for each person in the near future.
Reference
- Salata, O. V., Applications of nanoparticles in biology and medicine. Journal of nanobiotechnology. 2004, 2 (1).
CrossRef - Cai, W.; Gao, T.; Hong, H.; Sun, J., Applications of gold nanoparticles in cancer nanotechnology. Nanotechnology, science and applications. 2008, 2008 (1).
CrossRef - De Jong, W. H.; Borm, P. J., Drug delivery and nanoparticles: applications and hazards. International journal of nanomedicine. 2008, 3 (2), 133.
CrossRef - Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L., Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. Journal of Controlled Release. 2016, 235, 34-47.
CrossRef - Smola, M.; Vandamme, T.; Sokolowski, A., Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and nonrespiratory diseases. International journal of nanomedicine. 2008, 3 (1), 1.
- Scheuch, G.; Kohlhaeufl, M. J.; Brand, P.; Siekmeier, R., Clinical perspectives on pulmonary systemic and macromolecular delivery. Advanced drug delivery reviews. 2006, 58 (9), 996-1008.
CrossRef - Date, A. A.; Joshi, M. D.; Patravale, V. B., Parasitic diseases: liposomes and polymeric nanoparticles versus lipid nanoparticles. Advanced drug delivery reviews. 2007, 59 (6), 505-521.
CrossRef - Mitchison, D.; Fourie, P., The near future: improving the activity of rifamycins and pyrazinamide. Tuberculosis. 2010, 90 (3), 177-181.
CrossRef - Gurunathan, S.; Han, J. W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H., Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. BioMed research international. 2013, 2013.
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K., Establishment and characterization of a human acute monocytic leukemia cell line (THP‐1). International journal of cancer. 1980, 26 (2), 171-176.
CrossRef - Maurya, A.; Singh, A.; Kumar, M.; Umrao, J.; Kant, S.; Nag, V.; Kushwaha, R.; Dhole, T., Changing patterns and trends of multidrug-resistant tuberculosis at referral centre in Northern India: a 4-year experience. Indian journal of medical microbiology. 2013, 31 (1), 40.
CrossRef - Wh, O., Global tuberculosis control: surveillance, planning, financing. 2008.
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D. G., The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale research letters. 2009, 4 (12), 1409.
CrossRef - Murray, P. R.; Rosenthal, K. S.; Pfaller, M. A., Medical microbiology. Elsevier Health Sciences: 2015.
- Mura, S.; Couvreur, P., Nanotheranostics for personalized medicine. Advanced drug delivery reviews. 2012, 64 (13), 1394-1416.
CrossRef - Mirsaeidi, M., Personalized medicine approach in mycobacterial disease. International journal of mycobacteriology. 2012, 1 (2), 59-64.
CrossRef - Knol, A. B.; de Hartog, J. J.; Boogaard, H.; Slottje, P.; van der Sluijs, J. P.; Lebret, E.; Cassee, F. R.; Wardekker, J. A.; Ayres, J. G.; Borm, P. J., Expert elicitation on ultrafine particles: likelihood of health effects and causal pathways. Particle and Fibre Toxicology. 2009, 6 (1), 1.
CrossRef - Van Dong, P.; Ha, C. H.; Kasbohm, J., Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. International Nano Letters. 2012, 2 (1), 1-9.
CrossRef - Hu, Z.; Oskam, G.; Searson, P. C., Influence of solvent on the growth of ZnO nanoparticles. Journal of colloid and interface science. 2003, 263 (2), 454-460.
CrossRef - Becheri, A.; Dürr, M.; Nostro, P. L.; Baglioni, P., Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. Journal of Nanoparticle Research. 2008, 10 (4), 679-689.
CrossRef - Ghaedi, M.; Ghayedi, M.; Kokhdan, S. N.; Sahraei, R.; Daneshfar, A., Palladium, silver, and zinc oxide nanoparticles loaded on activated carbon as adsorbent for removal of bromophenol red from aqueous solution. Journal of Industrial and Engineering Chemistry. 2013, 19 (4), 1209-1217.
CrossRef - Gupta, P. S.; Sharma, V.; Pathak, K., Melt sonocrystallized piroxicam for oral delivery: particle characterization, solid state analysis, and pharmacokinetics. Expert opinion on drug delivery. 2013, 10 (1), 17-32.
CrossRef - Esmaeili, F.; Rajabnejhad, S.; Partoazar, A. R.; Mehr, S. E.; Faridi-Majidi, R.; Sahebgharani, M.; Syedmoradi, L.; Rajabnejhad, M. R.; Amani, A., Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharmaceutical Development and Technology. 2015, 1-7.
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A. G.; Reichel, C. A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F., Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Particle and fibre toxicology. 2008, 5 (1), 1.
CrossRef - Mitrano, D. M.; Lesher, E. K.; Bednar, A.; Monserud, J.; Higgins, C. P.; Ranville, J. F., Detecting nanoparticulate silver using single‐particle inductively coupled plasma–mass spectrometry. Environmental Toxicology and Chemistry. 2012, 31 (1), 115-121.
CrossRef - Majedi, S. M.; Lee, H. K.; Kelly, B. C., Chemometric analytical approach for the cloud point extraction and inductively coupled plasma mass spectrometric determination of zinc oxide nanoparticles in water samples. Analytical chemistry. 2012, 84 (15), 6546-6552.
CrossRef - Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.; Lin, M., Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157: H7. Journal of applied microbiology. 2009, 107 (4), 1193-1201.
CrossRef - Xiang, Q.; Meng, G.; Zhang, Y.; Xu, J.; Xu, P.; Pan, Q.; Yu, W., Ag nanoparticle embedded-ZnO nanorods synthesized via a photochemical method and its gas-sensing properties. Sensors and Actuators B: Chemical. 2010, 143 (2), 635-640.
CrossRef - Oshida, K.; Murata, M.; Fujiwara, K.; Itaya, T.; Yanagisawa, T.; Kimura, K.; Nakazawa, T.; Kim, Y.; Endo, M.; Kim, B.-H., Structural analysis of nano structured carbon by transmission electron microscopy and image processing. Applied Surface Science. 2013, 275, 409-412.
CrossRef - Prakash, P.; Gupta, N., Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian journal of physiology and pharmacology. 2005, 49 (2), 125.
- Singh, A. K.; Chakravarty, B.; Chaudhury, K., Nanoparticle-Assisted Combinatorial Therapy for Effective Treatment of Endometriosis. Journal of biomedical nanotechnology. 2015, 11 (5), 789-804.
CrossRef - Yin, H.; Langford, R.; Burrell, R., Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. Journal of Burn Care & Research. 1999, 20 (3), 195-200.
CrossRef - Mitrano, D.; Ranville, J. F.; Neubauer, K.; Shelton, C., ICP-Mass Spectrometry.
- Degueldre, C.; Favarger, P.-Y.; Wold, S., Gold colloid analysis by inductively coupled plasma-mass spectrometry in a single particle mode. Analytica Chimica Acta. 2006, 555 (2), 263-268.
CrossRef - Organization, W. H., Global tuberculosis report 2013. World Health Organization: 2013.
- Organization, W. H., The global prevalence of anaemia in 2011. 2015.
- Collins, F. S.; Varmus, H., A new initiative on precision medicine. New England Journal of Medicine. 2015, 372 (9), 793-795.
CrossRef - Banyal, S.; Malik, P.; Tuli, H. S.; Mukherjee, T. K., Advances in nanotechnology for diagnosis and treatment of tuberculosis. Current opinion in pulmonary medicine. 2013, 19 (3), 289-297.
CrossRef - AshaRani, P.; Low Kah Mun, G.; Hande, M. P.; Valiyaveettil, S., Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS nano. 2008, 3 (2), 279-290.
- Foldbjerg, R.; Dang, D. A.; Autrup, H., Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Archives of toxicology. 2011, 85 (7), 743-750.
CrossRef - de Moraes, A. C. M.; Lima, B. A.; de Faria, A. F.; Brocchi, M.; Alves, O. L., Graphene oxide-silver nanocomposite as a promising biocidal agent against methicillin-resistant Staphylococcus aureus. International journal of nanomedicine. 2015, 10, 6847.
CrossRef - Moussawi, R. N.; Patra, D., Modification of nanostructured ZnO surfaces with curcumin: fluorescence-based sensing for arsenic and improving arsenic removal by ZnO. RSC Advances. 2016, 6 (21), 17256-17268.
CrossRef - Ghorbani, H. R.; Mehr, F. P.; Pazoki, H.; Rahmani, B. M., Synthesis of ZnO Nanoparticles by Precipitation Method. Oriental Journal of Chemistry. 2015, 31 (2), 1219-1221.
CrossRef - Wrótniak-Drzewiecka, W.; Gaikwad, S.; Laskowski, D.; Dahm, H.; Niedojadło, J.; Gade, A.; Rai, M., Novel approach towards synthesis of silver nanoparticles from Myxococcus virescens and their lethality on pathogenic bacterial cells. Austin J Biotechnol Bioeng. 2014, 1 (1), 01-07.
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S. R. K.; Muniyandi, J.; Hariharan, N.; Eom, S. H., Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids and Surfaces B: Biointerfaces. 2009, 74 (1), 328-335.
CrossRef - Soto, K.; Garza, K.; Murr, L., Cytotoxic effects of aggregated nanomaterials. Acta Biomaterialia. 2007, 3 (3), 351-358.
CrossRef - Stebounova, L. V.; Adamcakova-Dodd, A.; Kim, J. S.; Park, H.; T O’Shaughnessy, P.; Grassian, V. H.; Thorne, P. S., Nanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation model. Particle and fibre toxicology. 2011, 8 (1), 1.
CrossRef - He, Y.; Du, Z.; Ma, S.; Liu, Y.; Li, D.; Huang, H.; Jiang, S.; Cheng, S.; Wu, W.; Zhang, K., Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. International journal of nanomedicine. 2016, 11, 1879.
CrossRef - Zhang, T.; Wang, L.; Chen, Q.; Chen, C., Cytotoxic potential of silver nanoparticles. Yonsei medical journal. 2014, 55 (2), 283-291.
CrossRef - Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A., Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Archives of toxicology. 2013, 87 (7), 1181-1200.
CrossRef - Reddy, K. M.; Feris, K.; Bell, J.; Wingett, D. G.; Hanley, C.; Punnoose, A., Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Applied physics letters. 2007, 90 (21), 213902.
CrossRef - Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P., Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Particle and fibre toxicology. 2009, 6 (1), 1.
CrossRef - Xu, M.; Li, J.; Iwai, H.; Mei, Q.; Fujita, D.; Su, H.; Chen, H.; Hanagata, N., Formation of nano-bio-complex as nanomaterials dispersed in a biological solution for understanding nanobiological interactions. Scientific reports. 2012, 2
CrossRef
- Levard, C.; Hotze, E. M.; Lowry, G. V.; Brown Jr, G. E., Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environmental science & technology. 2012, 46 (13), 6900-6914.
CrossRef - Ivask, A.; Bondarenko, O.; Jepihhina, N.; Kahru, A., Profiling of the reactive oxygen species-related ecotoxicity of CuO, ZnO, TiO2, silver and fullerene nanoparticles using a set of recombinant luminescent Escherichia coli strains: differentiating the impact of particles and solubilised metals. Analytical and bioanalytical chemistry. 2010, 398 (2), 701-716.
CrossRef - Casals, E.; Gonzalez, E.; Puntes, V., Reactivity of inorganic nanoparticles in biological environments: insights into nanotoxicity mechanisms. Journal of Physics D: Applied Physics. 2012, 45 (44), 443001.
CrossRef - Jafari, A.; Ghane, M.; Arastoo, S., Synergistic antibacterial effects of nano zinc oxide combined with silver nanocrystales. African Journal of Microbiology Research. 2011, 5 (30), 5465-5473.
- Jafari, A.; Majidpour, A.; Safarkar, R.; Noor-Allahi, M.; Arastoo, S., The Synthesis and Characterizes of Nano-Metallic Particles Against Antibiotic Resistant Bacteria, Isolated from Rasoul-e-Akram Hospital’s Patients, Tehran, Iran. Journal of Molecular Biology Research. 2016, 6 (1), 80.
CrossRef - Salah, R.; Michaud, P.; Mati, F.; Harrat, Z.; Lounici, H.; Abdi, N.; Drouiche, N.; Mameri, N., Anticancer activity of chemically prepared shrimp low molecular weight chitin evaluation with the human monocyte leukaemia cell line, THP-1. International journal of biological macromolecules. 2013, 52, 333-339.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









