Synthesis and Antibacterial Activity of 2-(2-(Cyclopropylmethoxy)Phenyl)-5-Methyl-1,3,4- Oxadiazole
A. Parameshwar1, V. Selvam2, Majid Ghashang3 and S. Guhanathan4
1,2Research and Development Centre, Bharathiar University, Coimbatore, Tamilnadu, India.
3Department of Chemistry, Faculty of Sciences, Najafabad Branch, Islamic Azad University, Najafabad, Esfahan, Iran.
4PG and Research Department of Chemistry, Muthurangam Government Arts College, Vellore- 632 002, India.
Corresponding Author E-mail: sai_gugan@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330355
Heterocyclic compounds containing the five-membered oxadiazole nucleus possess verity of biological activities. 1,3,4- oxadiazole moieties are important because of their versatile biological action. The present study depicts synthesis of some novel 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole was prepared from N'-acetyl-2-(cyclopropylmethoxy)benzohydrazide by using triphenylphosphine, triethylamine, carbon tetrachloride and acetonitrile at 100°C for 1h. The final compound was examined for its antibacterial activity.
KEYWORDS:Antibacterial activity; (bromomethyl)cyclopropane; gentamicin; hydrazine monohydrate; triphenylphosphine
Download this article as:| Copy the following to cite this article: Parameshwar A, Selvam V, Ghashang M, Guhanathan S. Synthesis and Antibacterial Activity of 2-(2-(Cyclopropylmethoxy)Phenyl)-5-Methyl-1,3,4- Oxadiazole. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Parameshwar A, Selvam V, Ghashang M, Guhanathan S. Synthesis and Antibacterial Activity of 2-(2-(Cyclopropylmethoxy)Phenyl)-5-Methyl-1,3,4- Oxadiazole. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=34010 |
Introduction
Compounds bearing oxadiazole moiety are of great importance Between five-membered heterocycles which have been recognized as privileged structures in medicinal chemistry. Four isomers of oxadiazoles are well-known which, amongst of them 1,3,4-oxadiazole nucleus is found in many therapeutic agents, such as Fenadiazole, Nesapidil, Furamizole, and Raltegravir (Figure 1) [1-4].
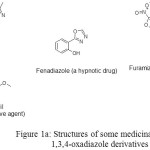 |
Figure 1a: Structures of some medicinally active 1,3,4-oxadiazole derivatives |
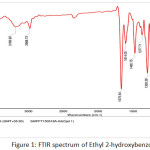 |
Figure 1: FTIR spectrum of Ethyl 2-hydroxybenzoate Click here to View figure |
 |
Figure 2: 1H-NMR spectrum of Ethyl 2-hydroxybenzoate Click here to View figure |
1,3,4-oxadiazole derivatives exhibited a wide variety of biological activities such as antimicrobial, antimitotic, antihypertensive, anticonvulsant, anti-inflammatory, and muscle relaxant [5-14]. The valuable medicinal properties of 1,3,4-oxadiazole make these compounds an important structural unit to be the subject of extensive research in recent years. Therefore, development of new structures of 1,3,4-oxadiazole would be highly desirable.
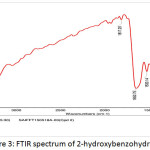 |
Figure 3: FTIR spectrum of 2-hydroxybenzohydrazide |
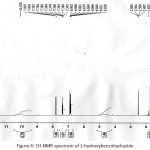 |
Figure 4: 1H-NMR spectrum of 2-hydroxybenzohydrazide Click here to View figure |
The common methods used for the synthesis of 1,3,4-oxadiazoles involves the cyclization of the carboxylic acid hydrazides with a variety of dehydrating reagents such as POCl3, concentrated sulfuric acid, phosphonium salts and Burgess-type reagents.
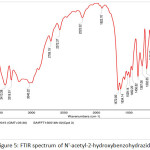 |
Figure 5: FTIR spectrum of N’-acetyl-2 hydroxybenzohydrazide |
 |
Figure 6: 1H-NMR spectrum of N’-acetyl-2-hydroxybenzohydrazide Click here to View figure |
Herein, we reported a five steps synthesis of 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole, starting from 2-hydroxybenzoic acid in good yield (Scheme 1).
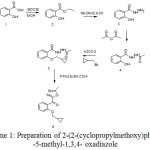 |
Scheme 1: Preparation of 2-(2-(cyclopropylmethoxy)phenyl) |
Reagents and Instrumentation
All reagents were purchased from Merck and Aldrich and used without further purification. The NMR spectra were recorded on a Bruker Avance DPX 400 MHz instrument. The spectra were measured in DMSO-d6 relative to TMS (0.00 ppm). Elemental analysis was performed on a Heraeus CHN-O-Rapid analyzer. TLC was performed on silica gel Polygram SIL G/UV 254 plates.
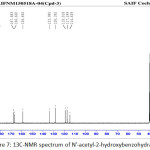 |
Figure 7: 13C-NMR spectrum of N’-acetyl-2-hydroxybenzohydrazide Click here to View figure |
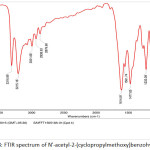 |
Figure 8: FTIR spectrum of N’-acetyl-2-(cyclopropylmethoxy)benzohydrazide Click here to View figure |
Experimental Procedure
Preparation of Ethyl 2-Hydroxybenzoate
Thionyl chloride (20 mL, 0.275 mmol) was added dropwise to a stirred solution of salicylic acid (10 g, 0.0724 mmol) in ethanol (50 mL) at 0°C and the mixture was stirred for 12h at 80°C. After completion of starting material (TLC monitoring), the reaction mixture was concentrated to remove the unreacted thionyl chloride and ethanol under reduced pressure. The residue was diluted with water and extracted with MTBE solvent . The organic layer was washed with an aqueous solution of NaHCO3 (10%), dried with sodium sulfate and concentrated under reduced pressure to obtain the pure product as a brown color liquid (Yield: 65%). 1H-NMR(400 MHz, DMSO-d6): δ = 10.58 (s, 1H, OH), 7.79 (d, J = 7.6 Hz, 1H), 7.51 (t, J = 8.0 Hz, 1H), 6.93-7.00 (m, 2H), 4.37 (q, J = 6.8 Hz, 2H), 1.34 (t, J = 7.2 Hz, 3H) ppm; IR: 3189 (O-H, phenol), 2988, 1675, 1614, 1480, 1300, 1249, 1216, 1091, 808, 756, 705 cm-1.
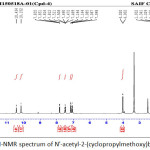 |
Figure 9: 1H-NMR spectrum of N’-acetyl-2-(cyclopropylmethoxy)benzohydrazide Click here to View figure |
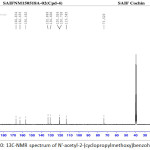 |
Figure 10: 13C-NMR spectrum of N’-acetyl-2-(cyclopropylmethoxy)benzohydrazide Click here to View figure |
Preparation of 2-Hydroxybenzohydrazide
Hydrazine monohydrate (20 mL) was added to a solution of ethyl 2-hydroxybenzoate (4 g, 0.024 mmol) in ethanol (100 mL) at room temperature and the reaction mixture was heated to 80°C and and stirred for 2h at same temperature. After completion of the reaction (TLC monitoring), ethanol was concentrated. To that residue ice cold water was added and stirred for 30 min until a white color solid was precipitated. The white solid was filtered and dried under vacuum (Yield 70%). 1H-NMR(400 MHz, DMSO-d6 ): δ = 10.10 (s,1H, OH), 7.78-7.81 (dd, J = 7.6, 1.6 Hz, 1H), 7.36 (td, J = 7.6, 1.6 Hz, 1H), 6.83-6.90 (m, 2H), 4.71 (s, 2H, NH2); IR: 3274 (N-H, amide), 1652, 1530, 1372, 1304, 1245, 1146, 1033, 957, 756 cm-1.
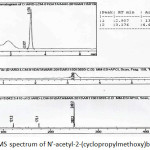 |
Figure 11: LC-MS spectrum of N’-acetyl-2-(cyclopropylmethoxy)benzohydrazide Click here to View figure |
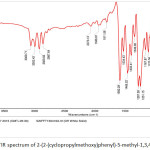 |
Figure 12: FTIR spectrum of 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole Click here to View figure |
Preparation of N’-acetyl-2-(cyclopropylmethoxy)benzohydrazide
To a solution of N‘-acetyl-2-hydroxybenzohydrazide (1 g, 0.0051 mmol) in acetonitrile:water (9:1) (10 mL) was added potassium carbonate (1.97 g, 0.015 mmol) and stirred for 15 min at room temperature. To this solution (bromomethyl)cyclopropane (0.83 g, 0.0061 mmol) was added dropwise and the reaction mixture heated at 90°C for 12 h. After1 consumption of starting material, acetonitrile was concentrated, then residue was diluted with water and extracted with ethyl acetate. Organic layer was dried over sodium sulphate and concentrated under reduced pressure to get off white semi solid. This crude was treated with hexane at 0°C and precipitated solid was filtered off to obtain the white solid (Yield 42%). 1H-NMR(400 MHz, DMSO-d6 ): δ = 10.43 (s,1H, NH), 10.15 (s, 1H, NH), 7.81-7.83 (dd, J = 7.6, 1.6 Hz, 1H), 7.46-7.51 (td, J = 7.2, 1.2 Hz, 1H), 7.04-7.16 (m, 2H), 4.02 (d, J = 7.2 Hz, 2H), 1.93 (s, 3H), 1.28-1.34 (m, 1H), 0.55-0.60 (m, 4H);13C-NMR (100 MHz, DMSO-d6): δ = 166.8, 162.6, 156.4, 132.8, 130.6, 120.9, 113.5, 73.2, 20.3, 9.9, 3.1; LC-MS: 249[M+H]; IR: 3316 (N-H, amide), 3215(N-H, amide), 3001, 2876, 1614, 1561, 1477, 1233, 1162, 1110, 986, 846, 745, 706 cm-1.
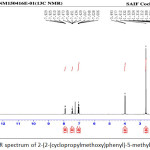 |
Figure 13: 1H-NMR spectrum of 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole Click here to View figure |
 |
Figure 14: 13C-NMR spectrum of 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole |
Preparation of 2-(2-(Cyclopropylmethoxy)Phenyl)-5-Methyl-1,3,4- Oxadiazole
To a solution of N‘-acetyl-2-(cyclopropylmethoxy)benzohydrazide (250 mg, 0.001 mmol) in acetonitrile (2.5 mL) was added a mixture of triphenylphosphine (341 mg, 0.0015 mmol), triethylamine (152 mg, 0.0015 mmol) and carbon tetrachloride (1.25 mL) under magnetic stirring. The reaction mixture was heated to 100°C and stirred for 1h at same temperature. After completion (TLC monitoring), the reaction mixture was completely concentrated and the crude product was purified by column chromatography eluted by ethyl acetate/hexane (5-10%) (Yield 52%). 1H-NMR(400 MHz, CDCl3-d6): 7.90-7.93 (dd, J = 7.6, 1.6 Hz, 1H), 7.42-7.47 (td, J = 8.0, 1.6 Hz, 1H), 7.00-7.07 (m, 2H), 3.99 (d, J = 6.4 Hz, 2H), 2.61 (s, 3H), 1.26-1.33 (m, 1H), 0.46-0.62 (m, 4H) ppm; 13C-NMR (100 MHz, CDCl3-d6): δ = 163.9, 163.5, 157.1, 132.7, 130.5, 120.8, 113.9, 113.7, 73.2, 11.0, 10.1, 3.0; LC-MS: 231[M+H]; IR: 3069, 3003, 2929, 2867, 1592, 1534, 1466, 1403, 1349, 1287, 1251, 1167, 1120, 1000, 761, 703 cm-1; Anal. Calcd. For C13H14N2O2; C, 67.81; H, 6.13; N, 12.17. Found: C, 67.11; H, 6.06; N, 12.03.
Antibacterial Activity
The in vitro antibacterial activity of 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole was analyzed by minimum inhibitory concentration (MIC) method. Escherichia coli and Klebsiella pneumonia as Gram negative bacteria and Staphylococcus aureus and Bacillus cereus as Gram positive bacteria were used to determine the antibacterial activity. Different concentrations of 50, 100, 150 and 200 μg/mL of the sample in N,N-dimethylformamide (DMF) were prepared in the test tubes. The lowest inhibitory concentration of the test compounds was determined by MIC method that shows the zone of inhibition after 18h. gentamicin was used as the standard drug in the assay
Results and Discussion
2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole was prepared in good yield via a five step synthesis starting from 2-hydroxybenzoic acid (scheme 1). 2-Hydroxybenzohydrazide was synthesized in two first steps esterification followed by hydrazinolysis. N‘-acetyl-2-hydroxybenzohydrazide was prepared by acylation reaction. The hydroxyl group then protected by the using of (bromomethyl)cyclopropane. An intramolecular dehydration-cyclization process using triphenylphosphine and triethylamine results in the synthesis of targeted molecular in good yield. The structures of the synthesized compounds at the end of each stage were also confirmed by NMR and FT-IR analysis.
The structure of 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole was confirmed on the basis of H and C-NMR, LC-MS, FT-IR and elemental analysis. The disappearance of NH protons in NMR as well as IR spectra confirmed the synthesis of oxadiazole ring.
The 1H-NMR spectra of 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole contained three signals in the aromatic region; a doublet of doublet (3J = 7.6, 4J = 1.6 Hz) assigned to proton H-1, the second triplet of doublet (3J = 8.0, 4J = 1.6 Hz) for proton H-3 and a multiplet for two protons H-2 and H-4. In the aliphatic region, the signal for CH2 was observed downfield in δ 3.99 either as a doublet. Methyl group appears as a singlet at δ = 2.61 and proton H-5 shows a multi-plate in δ = 1.26-1.33 (Scheme 2).
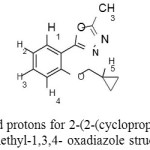 |
Scheme 2: assigned protons for 2-(2 (cyclopropylmethoxy)phenyl) -5-methyl-1,3,4- oxadiazole structure |
The antibacterial activity of the synthesized 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole was evaluated using two Gram-positive (Staphylococcus aureus and Bacillus cereus) and two Gram-negative (Escherichia coli and Klebsiella pneumonia) bacteria. Gentamicin was used as a reference drug. The MIC values of the compound were determined by using Muller-Hinton broth (Merck). The compound demonstrated good antibacterial activity against the Gram-negative bacteria (Escherichia coli and Klebsiella pneumonia) (Table 1).
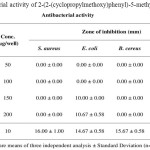 |
Table 1: antibacterial activity of 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole |
In summary, 2-(2-(cyclopropylmethoxy)phenyl)-5-methyl-1,3,4- oxadiazole was successfully synthesized in five steps in good yield and was used for antibacterial activity against Gram-positive and Gram-negative bacteria. In comparison with Gentamicin, the compounds show relatively a good antibacterial activity.
References
- Brandenberger, H.; Maes, A. R. Analytical Toxicology for Clinical, Forensic and Pharmaceutical Chemists, 1997.
- Adelstein, G. W.; Yen, C. H.; Dajani, E. Z.; Bianchi R, G. J. Med. Chem. 1976, 19, 1221–1225
CrossRef - Ichiro, H.; Yasuhiko, K.; Ryuzo, U.; Nippon Kagaku Zassi. 1967, 88, 574
CrossRef - Patel, K. D.; Prajapati, S. M.; Panchal, S. N.; Patel, H. D.; Review of synthesis of 1,3,4-oxadiazole derivatives. Synth. Commun. 2014, 44, 1859–1875
CrossRef - Fang, T.; Tan, Q.; Ding, Z.; Liu, B.; Xu, B.; Pd-Catalyzed Oxidative Annulation of Hydrazides with Isocyanides: Synthesis of 2‑Amino-1,3,4-oxadiazoles. Org. Lett. 2014, 16, 2342−2345
CrossRef - Dogan, H. N.; Duran, A.; Rollas, S.; Sener, G.; Uysal, M. K.; Gülen, D. Bioorg. Med. Chem. 2002, 10, 2893
- El-Emam, A. A.; Al-Deeb, O. A.; Al-Omar, M.; Lehmann. J. Bioorg. Med. Chem. 2014, 12, 5107
CrossRef - Lokanatha Rai, K. M.; Linganna, N.; Farmaco. 2000, 55, 389
CrossRef - Turner, S.; Myers, M.; Gadie, B.; Nelson, A. J.; Pape, R.; Saville, J. F.; Doxey, J. C.; Berridge, T. L. J. Med. Chem. 1988, 31, 902
CrossRef - Tyagi, M.; Kumar, A. Orient. J. Chem. 2002, 18, 125
- Chapleo, C. B.; Myers, P. L.; Smith, A. C.; Stillings, M. R.; Tulloch, I. F.; Walter, D. S. J. Med. Chem. 1988, 31, 7
CrossRef - Boschelli, D. H.; Connor, D. T.; Bornemeier, D. A.; Dyer, R. D.; Kennedy, J. A.; Kuipers, P. J.; Okonkwo, G. C.; Schrier, D. J.; Wright, C. D. J. Med. Chem. 1993, 36, 1802
CrossRef - Mullican, M. D.; Wilson, M. W.; Conner, D. T.; Kostlan, C. R.; Schrier, D. J.; Dyer, R. D. J. Med. Chem. 1993, 36, 1090
CrossRef - Omar, F. A.; Mahfouz, N. M.; Rahman, M. A. EurJ. Med. Chem. 1996, 31, 819
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









