Preparation, In-Vitro Bioactivity and Mechanical Properties of Reinforced 45S5 Bioglass Composite With HA-Zro2 Powders
Sunil Prasad1, Vikas Kumar Vyas1, Kumari Deepa Mani2, Md. Ershad1 and Ram Pyare1
1Department of Ceramic Engineering, Indian Institute of Technology (BHU), Varanasi-221005, India.
2Department of Zoology, Banaras Hindu University, Varanasi-221005, India.
Corresponding Author E-mail: sunilmnnit25@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330328
Bioglass(45S5)-Hydroxyapatite(HA)-Zirconia(ZrO2) biocomposites were prepared and heat treated at 1000,1100,1200°C for 5 hour. Simulated body fluid(SBF) is used to immersed these samples for 1,3,7,14,21 days. Samples were characterized before and after immersion in simulated body fluid by using FTIR, X-ray diffraction (XRD) and scanning electron microscope (SEM). FTIR were used to observed the formation of hydroxyapatite layer(HA) on the surface of these biocomposite samples. The pH of the SBF were measured by using pH meter at 1,3,7,14,21 days. Different techniques were used to measured various mechanical properties and it was found to increase with increasing HA(10,20,30,40 wt%) and ZrO2(5,10,15,20 wt%) content.
KEYWORDS:Bioglass(45S5); Hydroxyapatite(HA); Zirconia; Biocomposites; Simulated body fluid(SBF); FTIR spectrometry
Download this article as:| Copy the following to cite this article: Prasad S, Vyas V. K, Mani K. D, Ershad M, Pyare R. Preparation, In-Vitro Bioactivity and Mechanical Properties of Reinforced 45S5 Bioglass Composite With HA-Zro2 Powders. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Prasad S, Vyas V. K, Mani K. D, Ershad M, Pyare R. Preparation, In-Vitro Bioactivity and Mechanical Properties of Reinforced 45S5 Bioglass Composite With HA-Zro2 Powders. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33240 |
Introduction
Hench glass 45S5[1] has been extensively used to repair hard and soft tissue bone because of their excellent bioactive properties. However, an incomplete conversion into a bone-like material undergo by these bioactive materials which severely limits their use in biomedical application[2]. 45S5 Bioglass is generally used in making various biomedical devices, such as middle ear, dental implants etc. Due to the brittleness nature of 45S5 bioglass and relatively its low strength, the application in non-load bearing situations is limited[3]. This silicate-based bioactive glass has very low degradation and It has been found that remains in our body approximately more than one year after implantation[4]. This bioglass(45S5) does not alone provide sufficient bioactivity and mechanical strength due to this reason we need to reinforced this bioactive glass with increasing content of HA and ZrO2.
Chemical composition of HA has similar to inorganic mineral of our bone and teeth[5-6]. It has excellent biocompatibility and bioactivity. HA offers excellent biocompatibility, bioactivity [7], low density, low compressive strength and low hardness. It has also relatively low mechanical properties. Therefore the use of HA as a load bearing implant teeth is very limited. Due to these limitation there is a need to strengthening of HA without loosing its biocompatibility[8]. It was found that zirconia (ZrO2) possess high mechanical strength and very low toxicity [9-10]. For this reason (ZrO2) is widely used as a biomaterial for hip prosthesis [11-12], tooth crowns[13] and dental implants[14] and it was formed as a new bone restoring material in future. Sintered zirconia (ZrO2) has very high mechanical strength than a cortical bone therefore new class zirconia is used as a new bone restoring material[15-16]. Because of difference in strength, the amount of stress and frequent bone fracture may occur resulting in bonding to a host bone. Due to the poor affinity to cells and tissues of zirconia (ZrO2), there is a need to make composite by HA and ZrO2 mixed together in order to combine the biocompatibility of HA, high toughness and strength of ZrO2. It has been observed zirconia retain high mechanical strength and toughness with HA without affecting the biocompatibility of HA[17-21]. The changes in physico-chemical properties of the material is due to decomposition of HA. Performance of implant material is affected by change in its density, solubility, resorption and biocompatibility during implantation in a living body. Therefore HA decay is an important problem, both from scientific and application point of view. It has been found that calcium present in hydroxyapatite can react with ZrO2 transformed zirconia into cubic form which make it tough by this transformation. [22].
Further it was observed that HA-ZrO2 composites have very improved strength and toughness as compared to monolithic HA itself [23-26]. It is well known that P2O5 act as a strong glass network foemer. Covalent bond is formed between PO4 tetrahedra structures in chains or rings by bridging oxygens[27]. It is also known that Na2O and CaO were act as glass network modifiers and formed non-bridging oxygens (NBO) into the glass network[28]. In environment Zr present as a common trace element or metallic Zr(IV) normally present in human bone and tissue as low in the range of 2–10mg/kg body weight with an estimated average daily intake in humans of ~2.6 mg. The toxicity of Zr has been assessed low to moderate in animals [29]. Ceramics can be devided into two main groups that has been used as an implant material i.e. bioinert and bioactive. Bioinert ceramics, such as zirconia (ZrO2) show no interaction with the surrounding and living tissue. However, bioactive ceramics such as calcium phosphates are forming bonds with living hard and soft tissue. Since it has the main inorganic constituent of bones and teeth. HA is the most interesting bioactive ceramics[30].
Partially stabilized zirconia (PSZ) is bioinert and has highest strength and fracture strength was mixed with HA to obtained a bioactive implants with increased mechanical properties[31-32]. Similarly other oxides, such as alumina(Al2O3) [33], titania(TiO2)[34], and yttria(Y2O3)[35] were studied to prepare HA composites in different structures via several methods.
In present investigation to obtained high load bearing implants of BG-HA-ZrO2 composites were prepared with increasing HA and ZrO2 in base bioactive glass to make biocomposite. Structural in-vitro and mechanical properties of these biocomposite material were studied at different sintering temperature.
Materials and Methods
Synthesis of BG-HA-Zro2 Composites
45S5 Bioglass was prepared by using analytical grade quartz, calcium carbonate, sodium carbonate and ammonium dihydrogen orthophosphate by melting at 1400-1410°C with air as atmosphere and annealed in air oven at 500-550°C. Hydroxyapatite was prepared by solgel technique Fig.1. Bioglass (45S5), HA and ZrO2 powder were milled and mixed by ball milling about 4 hours. Samples were placed and sintered in furnace at 1000,1100 and 1200°C for 4 hours, rate of heating and cooling is at 5°C/minute. Uniaxial pressure of 100 MPa was applied to form rectangular bar shape sample of 4mm×6mm×40mm size by using die of size 55mm×10mm. The composition of bioglass and biocomposites were illustrated in Table 1.
 |
Figure 1: Flow chart of hydroxyapatite preparation by the sol-gel route. Click here to View figure |
Table 1: Composition of Bioactive Glass and Biocomposites (BHZ1, BHZ2, BHZ3, BHZ4).
| Composition (wt %) | ||||
| BG (45S5) | 45 SiO2 | 24.5 Na2O | 24.5 CaO | 6 P2O5 |
| Biocomposite Samples | BG (45S5) | HA | ZrO2 | |
| BHZ1 | 85 | 10 | 5 | |
| BHZ2 | 70 | 20 | 10 | |
| BHZ3 | 55 | 30 | 15 | |
| BHZ4 | 40 | 40 | 20 | |
XRD Characterization
Cu-Kα radiation at 40kV/40mA was used to evaluate the presence of different phases through X-ray diffraction (XRD, Bruker D8) analysis. The scanning range of 2θ angle was from 20° to 70° at scanning rate of 0.03809°/s with step size of 0.02°. The diffractograms were compared with JCPDS cards.
In-Vitro Analysis of Biocomposites Samples
At 37°C, the in vitro bioactivity evaluation of the biocomposite were determined by sample immersion in simulated body fluid (SBF) solutions for 1 to 21 days. SBF were prepared to determine in-vitro analysis of biocomposite samples. The SBF solution was prepared by Kokubo method [36] and compared ion concentration (mM/litre) of SBF with human blood plasma solution which are shown in table 2. The composite samples in the form of circular pellet having the size of 1 cm diameter were immersed in SBF at 37°C. pH of the SBF solutions was measured by using digital pH meter (model-1611,ESICO-USA) for 1,3,7,14,21 days time periods.
Table 2: The ions concentration of SBF solution and human blood plasma (mM/litre).
| S.No. | Ion | Simulated body fluid solution | Human blood plasma |
| 1 | Na+ | 142.1 | 142.1 |
| 2 | K+ | 5.2 | 5.2 |
| 3 | Mg2+ | 1.6 | 1.6 |
| 4 | Ca2+ | 2.6 | 2.6 |
| 5 | Cl– | 147.9 | 103.1 |
| 6 | HCO3– | 4.2 | 27.0 |
| 7 | HPO42- | 1.1 | 1.1 |
| 8 | SO42- | 0.6 | 0.6 |
Structural Analysis of Biocomposites by FTIR Transmittance Spectroscopy
Immersion of biocomposites in SBF before and after, formation of hydroxy carbonated apatite layer was formed on the surface and determined by FTIR (Shimadzu–8400S,Japan) used to record at the room temperature in the spectral range 4000-400 cm-1.
Morphological Analysis Using Scanning Electron Microscope(SEM) and EDS
The surfaces of bioactive composite were analyzed before and after immersion in SBF. These biocomposite samples were coated with gold plate before scanning of the sample with SEM. A scanning electron microscope (SEM–EV018,Carl Zeiss,UK) was used to analysis the surface microstructure of these samples before and after immersion in SBF. Elemental analysis were carried out by using energy dispersion spectrometry (EDS–51N1000,Oxford,UK) [37-41].
Physical and Mechanical Properties
Archimedes Principle was used to determine the density (Sartorius, Model: BP221S,USA) of these samples. UTM Machine (Tinius Olsen, H10KL, India) have been used to determine Compressive and toughness strength. Vickers microhardness test was calculated using a micro hardness tester (HMV-2, Shimadzu, Japan) with a diamond indenter at 1.968N load for 30s. The average microhardness was calculated by using formula. HV=0.001854P/d2
Result
XRD Analysis of Bioactive Composite Sample
The XRD pattern of sample BHZ1, BHZ2, BHZ3 and BHZ4 were presented in Fig.2, heated at 1000°C contained hydroxyapatite and tetragonal-zirconia phases. Upon heat treatment at higher temperatures i.e. 1100°C and 1200°C, the zirconia and wollastonite gradually transformed into zirconium silicate(zircon) respectively. Before and after soaking in SBF for various time periods i.e.1, 3, 7, 14 and 21 days, typical XRD patterns were obtained on the surfaces of biocomposites. XRD patterns of the biocomposite sample with HA(10,20,30,40 wt%) and ZrO2(5,10,15,20 wt%) is shown in the Fig.2(a-d). After immersion in SBF, crystalline peaks appear in the XRD patterns which indicates the formation of a crystalline layer on the surface of the biocomposites. Initially well-defined hydroxyapatite (HA) peaks develop at (2θ) values of 23-27° of these composites after 3 days of soaking in SBF. and ZrO2 peaks develop at (2θ) values of 30-35°after 7 days of immersion.
 |
Figure 2: XRD pattern of biocomposite sample BHZ1, BHZ2, BHZ3 and BHZ4 after SBF treatment (1,3,7,14,21) days. Click here to View figure |
The reaction between the HA and the ZrO2 forms TCP and cubic ZrO2 as follows [42]:
Ca10(PO4)6(OH)2 → 3Ca3P2O8 + CaO + H2O (1)
CaO + ZrO2 → -ZrO2 or CaZrO3 (2)
Hydroxyapatite transforms into TCP by releasing calcium (or CaO) and water vapour as shown in equation (1). HA phase is not stable, and TCP is the major phase. According to reaction (2), the reaction continues, a lot of CaO is released, thus cubic-ZrO2 are formed.
Transmission FTIR Analysis of Biocomposite Sample
FTIR spectra bands of BHZ1 sample illustrated in Fig.3(a) for 3 ,7,14 and 21 days treated with SBF. At 516 and 639 cm-1 P–O bending (crystalline) and P–O bending (amorphous) were formed. At 916 cm−1 C–O stretching band shown which indicates the formation of HCA layer. The bands at about 1512 and 1695 cm−1 were related to C–O (Stretch) and C=O (Stretch) modes respectively, and the broad band at about 3748 cm−1 are formed due to O–H groups on the surface. The samples in SBF for prolonged period indicates the similar response with small decrease in the intensities of the bands due to the formation of HCA layer. Further almost similar vibrations in all specimens (BHZ2, BHZ3, BHZ4) in Fig.3(b),(c),(d) which confirm apatite formation, these bands are also shown in table 3.
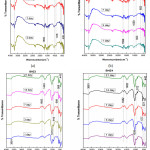 |
Figure 3: FTIR of the biocomposite sample BHZ1, BHZ2, BHZ3 and BHZ4 after immersion in SBF treatment for (1,3,7,14,21) days. Click here to View figure |
Table 3: FTIR spectra bands of BHZ1, BHZ2, BHZ3, BHZ4 sample.
| Sample | P–O (crystalline) | P–O (amorphous) | C–O (Stretch) | C=O (Stretch) | O–H groups |
| BHZ1 | 516 | 639 | 916, 1512 | 1695 | 3748 |
| BHZ2 | 516 | 652 | 923, 1404 | 1699 | 3745 |
| BHZ3 | 566 | 689 | 912, 1493 | 1682 | 3681 |
| BHZ4 | 521 | 577 | 912, 1482 | 1682 | 3681 |
The samples in SBF for prolonged period shows the same response with small decrease in the intensities of the bands that resulted in the formation of HCA layer.
Zr can be react in two ways.
Zr as glass network modifier: Possibility of some splitting in Si-O-Si bonds leads to lower connectivity of the silicate network with increase in O/Si ratio in BHZ with respect to 45S5. This phenomenon results in decrease of bridging oxygens and with an increase in non-bridging oxygens. However, after the incorporation of Zr, we found a significant reduction in the number of non-bridging oxygen in the FTIR spectra of the dried gels.
Zr as glass former: Zr may even act as a glass former with [ZrO4 ]4- units occupying some positions of [SiO4]4- tetrahedra. The extent of shifting the 29Si, resonance for a given tetrahedron is positive due to each covalent Si– O–Zr bridge [43].
Ph Analysis of Biocomposite Samples
Change in pH of biocomposite (BHZ1, BHZ2, BHZ3 and BHZ4) were presented in Fig.4 after immersing all samples in SBF for different time period. It was found that pH has increased upto 7 days due to the fast release of alkali ions (Na+) and alkaline earth ions (Ca2+) and its exchange with H+ or H3O+ ions in the simulated body fluid (SBF) solution. There is increase in OH– ions which increase the pH of the solution and breaking of Si-O-Si bonds which hold the glass structure together. Thus there is formation of silanols which decrease the pH of the solution after 7 to 21 days. Soaking in SBF leads to the formation of an apatite layer on the surface of the composite samples depends upon the morphological properties of biocomposite sample[45-46]. In vitro hydroxyapatite formation on body fluid inside simulated body fluid (SBF)[47] has four proposed stages. First: Fast cation exchange of Na+ and Ca2+ with H+ in solution, forming silanol bonds (Si-OH) on the glass surface i.e. Si-O-Na+ + H+ + OH– → Si-OH + Na+ (aq) + OH– , Second: A silica-rich (cation vacant) regions forms near the glass surface results increase in pH. Phosphate is also dissipated from the glass if available in the composition. Third: High local pH splits the Si-O-Si bonds and release OH– which causes an attack to the silica glass network. Soluble silica dissolves in the solution in the form of Si(OH)4 and leaving more Si-OH (silanols) at the glass-solution interface: Si-O-Si + H2O → Si-OH + OH-Si, Fourth: Condensation of Si-OH groups near the glass surface due to repolymerization of the silica-rich layer.
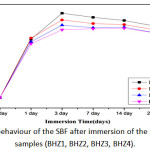 |
Figure 4: pH behaviour of the SBF after immersion of the biocomposite samples (BHZ1, BHZ2, BHZ3, BHZ4). Click here to View figure |
SEM and EDS Analysis of Biocomposite Sample After Soaking in SBF
After in vitro studies the surface morphology and hydroxyapatite layer formation on the surface of BHZ samples are shown using (SEM–EV018,Carl Zeiss,UK) Fig.5(a-d). Before in vitro studies, a compact and uniform morphology is viewed for BHZ sample. Where BHZ samples obtained after 21days of immersion in SBF, clear spherical apatite crystals were seen on the surface, which matches with the measured results [48]. After in vitro studies, due to introduction of hydroxyapatite and ZrO2 in BHZ sample a strong formation of HA layer on the sample surface took place. The above results shows that reactivity of BHZ sample is high in SBF samples. Fig. 5(e-h) shows the cross-sectional view and corresponding energy dispersive (EDS) spectra of BHZ sample after in vitro studies by using instrument (EDS–51N1000,Oxford,UK). The observed results show an embedded spherical hydroxyapatite layer on the surface of the biocomposite. Calcium and phosphate on the biocomposite surface is confirmed by EDS spectra of BHZ sample, which shows the presence of strong in vitro bioactivity.
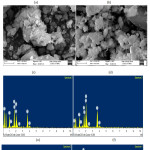 |
Figure 5: SEM of biocomposite samples after SBF (a-d) and EDX of biocomposite samples (e-h). Click here to View figure |
Mechanical Properties of BHZ Biocomposites
Fig.6(a) shows the density and microhardness of biocomposite, this result shows increase in hydroxyapatite and zirconia content which leads to increase in density and hardness value. Fig.6(b) and (c) shows the strength and fracture toughness of 45S5, BHZ1, BHZ2, BHZ3 and BHZ4 samples as a function of sintering temperature and found to increase with increasing sintering temperature. In Fig.6(b) the strength of the 45S5, BHZ1, BHZ2, BHZ3 and BHZ4 composite samples was about 56MPa,65MPa,72MPa,88MPa,109MPa respectively. Sintering at 1000°C lowering the strength of the bodies due to insufficient compactness. With increasing sintering temperature to 1200°C the strength value increases due to complete compactness. The fracture toughness show significant changes with the changing sintering temperature. The fracture toughness of the samples sintered at 1000°C, 1100°C and 1200°C were BHZ1(0.672,0.691,0.715MPa.m1/2),BHZ2(0.691,0.714,0.736MPa.m1/2),BHZ3(0.699,0.725,0.753MPa.m1/2) and BHZ4(0.715,0.732,0.756MPa.m1/2) respectively. Therefore, due to its superior strength and toughness, the samples sintered at 1200°C were chosen for a set of structural examinations.
 |
Figure 6a: Density and Vickers microhardness (b) Compressive strength (c) Fracture toughness of biocomposites (BHZ1, BHZ2, BHZ3 and BHZ4) sample. Click here to View figure |
Conclusion
The increasing amount of hydroxyapatite (10,20,30,40wt%) and zirconia (5,10,15,20wt%) reinforced with base bioactive glass upto the limit increase the bioactivity. Beyond 20wt%, addition of ZrO2 causes a decrease in maxima of the pH of SBF solution containing immersed samples. In present investigation it was found that biocomposite having 40wt% HA, 20wt%ZrO2 and 40wt% bioactive glass have highest bioactivity, density, microhardness, compressive strength and fracture toughness. Thus, we can say that reinforcement of HA+ZrO2 in the 45S5 bioactive glass would be a good bioactive materials.
Acknowledgment
The authors gratefully thanks to Department of Ceramic Engineering, IIT (BHU) and Central Instrument Fascility, IIT (BHU) Varanasi, India for providing required facilities for the present research work. The present work was supported by Rajiv Gandhi National Fellowship, University Grant Commission , New Delhi, India
Reference
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K.; J. Biomed. Mater. Res.1971, 2, 117.
CrossRef - Hench, L.L.; Bioceramics: From concept to clinic. J Am Ceram Soc.1991, 74,1487-1510.
CrossRef - Cao, W.; Hench, L.L.; Bioactive Materials. Ceramics International 1996, 22, 493-507.
CrossRef - Hamadouche, M; Meunier, A.; Greenspan,D.C.; Blanchat,C.; Zhong, J.P.; Torre, G.P. La; Sedel, L. J. Biomed. Mater. Res. 2001, 54,560.
CrossRef - Jarcho, M; Kay, JF; Gumaer, KI; Doremus, RH; Drobeck, HP; Tissue, cellular and subcellular events at a bone-ceramic hydroxylapatite interface. J. Bioeng. 1977,1(2), 79–92.
- Nayak, AK.; Hydroxyapatite synthesis methodologies: An overview. Int J ChemTech Res. 2010,2(2),903-7.
- Hench, L.L.;Wilson; J. An Introduction to Bioceramics. World Scientific 1993, 386.
CrossRef - Balani, K; Lahiri, D; Keshri, AK; Bakshi, SR; Tercero, JE; Agarwal, A; The nano-scratch behavior of biocompatible hydroxyapatite reinforced with aluminum oxide and carbon nanotubes. Jom 2009,61(9), 63–6.
CrossRef - Sollazzo, V; Pezzetti, F; Scarano, A; Piattelli, A; Bignozzi, CA; Massari, L; Zirconium oxide coating improves implant osseointegration in vivo. Dent Mater 2008, 24, 357–61.
CrossRef - Albrektsson, T; Hansson, HA; Ivarsson, B; Interface analysis of titanium and zirconium bone implants. Biomaterials 1985,6, 97–101.
CrossRef - Masonis, JL; Bourne, RB; Ries, MD; McCalden, RW; Salehi, A; Kelman, DC; Zirconia femoral head fractures: a clinical and retrieval analysis. J Arthroplast 2004,19, 898–905.
CrossRef - DeAza, AH; Chevalier, J; Fantozzi, G; Schehl, M; Torrecillas, R; Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials 2001,23, 937–45.
CrossRef - Kong, Y; Yang, Z; Zhang, G; Yuan, Q; Friction and wear characteristics of mullite, ZTM and TZP ceramics. Wear 1998, 218,159–66.
CrossRef - Kohal, RJ; Wolkewitz, M; Mueller, C; Alumina-reinforced zirconia implants: survival rate and fracture strength in a masticatory simulation trial. Clin Oral Implants Res. 2010 ,21, 1345–52.
CrossRef - Atilgan, S; Erol, B; Yardimeden, A; Yaman, F; Ucan, MC; Gunes, N; A three dimensional analysis of reconstruction plates used in different mandibular defects. Biotechnol Biotechnol Equip. 2010,24, 1893–6.
CrossRef - Nisitani, H; Kim, YH; Goto, H; Nishitani, H; Effects of gage length and stress concentration on the compressive strength of a unidirectional CFRP. Eng Fract Mech 1994, 49, 953–61.
CrossRef - Li, J; Liao, H; Hermansson, L; Sintering of partially-stabilized zirconia and partially-stabilized zirconia—hydroxyapatite composites by hot isostatic pressing and pressureless sintering. Biomaterials 1996, 17(18), 1787–90.
CrossRef - Silva, V V.; Lameiras, FS; Domingues, RZ; Microstructural and mechanical study of zirconia-hydroxyapatite (ZH) composite ceramics for biomedical applications. Compos Sci Technol 2001 , 61, 301–10.
CrossRef - Delgado, JA; Morejo´n, L; Marti´nez, S; Ginebra, MP; Carlsson, N; Ferna´ndez, E; Zirconia-toughened hydroxyapatite ceramic obtained by wet sintering. J Mater Sci Mater Med 1999,10(12), 715–9.
CrossRef - Kong, Y-M; Kim, S; Kim, H-E; Lee, I-S.; Reinforcement of Hydroxyapatite Bioceramic by Addition of ZrO2 Coated with Al2O3. J Am Ceram Soc. 2004, 82(11), 2963–8.
CrossRef - Takagi, M; Mochida, M; Uchida, N; Saito, K; Uematsu, K.; Filter cake forming and hot isostatic pressing for TZP-dispersed hydroxyapatite composite. J Mater Sci Mater Med 1992 ,3(3),199–203.
CrossRef - Li, J; Hermansson, L; Söremark, R; High-strength biofunctional zirconia: mechanical properties and static fatigue behaviour of zirconia-apatite composites. J Mater Sci Mater Med 1993 , 4(1),50–4.
CrossRef - Zhang, J; Iwasa, M; Kotobuki, N; Tanaka, T; Hirose, M; Ohgushi, H; Fabrication of Hydroxyapatite Zirconia Composites for Orthopedic Applications. J Am Ceram Soc. 2006 ,89(11) ,3348–55.
CrossRef - Khalil, KA; Kim, SW; Kim, HY; Consolidation and mechanical properties of nanostructured hydroxyapatite(ZrO2+3mol% Y2O3) bioceramics by high-frequency induction heat sintering. Mater Sci Eng A. 2007 ,456(1-2), 368–72.
CrossRef - Rapacz-Kmita, A; Ślósarczyk, A; Paszkiewicz, Z; Mechanical properties of HAp–ZrO2 composites. J Eur Ceram Soc. 2006, 26(8), 1481–8.
CrossRef - Wu; Jenn-Ming; T-SY; Sintering of hydroxylapatite-zirconia composite materials. J Mater Sci. 1988, 23(10),3771–7.
CrossRef - Little flower ,G; Reddy ,M. Srinivasa; Reddy ,M.V. Ramana; Veeraiah, N.; Naturforsch ,Z.; A: Phys. Sci. 2007, 62, 315.
- Reddy,M. Srinivasa ; NagaRaju ,G.; Nagarjuna,G.; Veeraiah, N. ; J. Alloys Comp. 2007,41, 438.
- Ghosh, S. ; Sharma, A.; Talukder, G.; Biol. Trace Elem. Res. 1992, 35, 247.
CrossRef - Vallet-Regi, M; González-Calbet, J.M.; Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31.
CrossRef - Shen, Z.J.; Adolfsson, E.; Nygren, M.; Gao, L.; Kawaoka, H.; Niihara, K.; Densehy- droxyapatite–zirconia ceramic composites with high strength for biological applications. Adv.Mater. 2001,13, 214–216.
CrossRef - Silva,V.V.; Domingues, R.Z.; Lameiras, F.S.; Microstructural and mechanical study of zirconia-hydroxyapatite(ZH) composite ceramics for biomedical applications. Compos. Sci. Technol. 2001, 61, 301–310.
CrossRef - Zhang, C.; Zhang, X.; Liu, C.; Sun, K.; Yuan, J.; Nano-alumina/hydroxyapatite composite powders prepared by in-situchemical precipitation. Ceram.Int. 2016, 42, 279–285.
CrossRef - Milella, E.; Cosentino, F.; Licciulli, A.; Massaro, C.; Preparation and characterisation of titania/ hydroxyapatite composite coatings obtained by sol–gel process. Biomaterials 2001, 22, 1425–1431.
CrossRef - Parente, P.; Sanchez-Herencia, A.J.; Mesa-Galan, M.J.; Ferrari, B.; Functionalizing Ti-Surfaces through the EPD of hydroxyapatite/nanoY2O3. J.Phys.Chem., 2012, B117,1600–1607.
- Kokubo, T; Kushitani, H; Sakka, S; Kitsuqi,T; Yamamuro,T.; Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramics A-W. Journal of Biomedical Materials Research 1990, 24, 721-734.
CrossRef - Tampieri, A.; D’Alessandro,T.; Sandri,M.; Sprio,S.; Landi,E.; Bertinetti,L.; Panseri,S.; Pepponi, G.; Goettlicher,J.; Bañobre-López, M.; Rivas, J.; Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8 (2), 843–851.
CrossRef - Fujibayashi, S.; Neo, M.; Kim, H.M.; Kokubo, T. ; Nakamura, T.; A comparative study between in vivo bone in growth and in vitro apatite formation on Na2O–CaO–SiO2 glasses. Biomaterials 2003, 24, 1349–1356.
CrossRef - Clupper, D.C.; Gough, J.E.; Embanga, P.M.; Notingher,I.; Hench, L.L.; Hall ,M.; Bioactive evaluation of 45S5 bioactive glass fibres and preliminary study of human osteoblast attachment. J. Mater. Sci. Mater. Med. 2004, 15, 803–808.
CrossRef - Kokubo, T.; Surface chemistry of bioactive glass-ceramics. J. Non-Cryst. Solids 1990, 120,138–151.
CrossRef - Kim, H.; Miyaji, F.; Kokubo, T.; Kokubo (1995). Bioactivity of Na2O–CaO–SiO2 glasses. J. Am. Ceram. Soc. 1995, 79 (9), 2405–2411.
CrossRef - Wu, J.M.; Yeh, T.S.; Sintering of hydroxylapatite–zirconia composite materials. J. Mater. Sci. 1988 , 23, 3771.
CrossRef - Barbieri, L.; Cannillo, V.; Leonelli, C.; Montorsi, M.; Mustarelli, P.; Siligardi, C.; Experimental and MD simulations study of CaO–ZrO2– SiO2 glasses. J.Phys.Chem.B 2003,107, 6519–6525.
CrossRef - Greenspa, DC; L.L. Hench; Chemical and mechanical behavior of bioglass coated alumina. J Biomed Materials Res 1976, 10, 503-509.
CrossRef - Satoshi, H; Kanji, T; Chikara, O; Akiyoshi, O; Mechanism of Apatite Formation on a Sodium Silicate Glass in a Simulated Body Fluid. J Am Ceram Soc 1999, 82, 2155-2160.
- Toshihiro, K; Yoshimasa, H; Masayuki, N; Mitsuo, N; Apatite Formation on Calcium Phosphate Invert Glasses in Simulated Body Fluid. J AmCeram Soc. 2001 ,84, 450-452.
- Clark, AE; Pantano, CG; Hench, L.L.; Auger spectroscopic analysis of Bioglass corrosion films. J Am Ceram Soc.1976, 59, 37-39.
CrossRef - P.Li, Ohtuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; Nakamura, T.; Yamamuro, T.; Effects of ions in aqueous media on hydroxyapatite induction by silica gel and its relevance to bioactivity of bioactive glasses and glass-ceramics. J. Appl. Biomater. 1993, 4, 221–229.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









