Investigation of Rotations in Isomerism Forms if A Ylide of Theophylline: Dynamic 1H NMR Study
Sayyed MostafaHabibi-Khorassani1, Mehdi Shahraki1, Eideh Mofarrah1 and Elham Mofarrah2
1Department of Chemistry, University of Sistan and Baluchestan, P. O. Box 98135-674, Zahedan, Iran.
2Department of Chemical Engineering, Amirkabir University of Technology, Tehran, Iran.
Corresponding Author E-mail: smhabibi@chem.usb.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/330316
The internal rotations around the some within a ylide involving theophylline has been studied using the dynamic 1H NMR spectroscopic technique in different temperatures. The kinetic and activation quantities were calculated. Arrhenius, Eyring and classic methods were applied for isomers. compared for carbon-carbon double, carbon-carbon single bond for Z isomer. The results obtained by classic method was different with data calculated from Arrhenius and Eyring methods while these methods were consistent together.
KEYWORDS:Ylide; Isomerism; Dynamic 1H NMR; Activations Parameters
Download this article as:| Copy the following to cite this article: MostafaHabibi-Khorassani S, Shahraki M, Mofarrah E. Mofarrah E.Investigation of Rotations in Isomerism Forms if A Ylide of Theophylline: Dynamic 1H NMR Study. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: MostafaHabibi-Khorassani S, Shahraki M, Mofarrah E. Mofarrah E.Investigation of Rotations in Isomerism Forms if A Ylide of Theophylline: Dynamic 1H NMR Study. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33059 |
Introduction
Ylides are important compound in the synthesis of plenty products with the various applications in pharmacology and medicine [1-7]. Moreover, theophylline characterized 1, 3-dimethylxanthine, is known as an impressive bronchodilator drug [8]. This compound has been used for over half a century in the treatment of asthma and remains the most widely prescribed anti-asthma treatment in the whole world [9]. This compound also used in treatment chronic pulmonary obstructive disease (COPD) [10-11-12-13], anti-Inflammatory [9, 14], immunomodulatory [9], Airway smooth muscle effects [15-16],effectsinfant apnea [17, 18]. Theophylline is naturally in green and black tea, coffee, and cocoa [19].
Theophylline in structure of ylides has significant dynamic 1H NMR effects. So, these effects afford important information regarding the rotational isomers [2, 20-25].
In this work, the dynamic 1H NMR effects on the titled compound prepared of the reaction between triphenylphosphine 1 and dimethylacetylendicarboxylaten 2 in the presence of theophylline 3 has been described. The synthesis reaction has been previously reported [26]. For these reasons, three methods for determining the activation parameters are applied [27].
Chemicals and Instruments
Tiphenylphosphine 1, dimethyl acetylendicarboxylate 2 and theophylline 3 obtained from Fluka (Buchs, Switzerland) are used in analytical grade,. The extra pure solvents including ether and acetone-d6 (supplied by Merk, Darmstadt Germany) are used in this work. The 1HNMR spectra were recorded on a FTNMR 400.22 MHz-BRUKER-Avance III.
Results and Discussion
The presence of two isomers Z nd E for the synthesized ylide 4 was confirmed by the 1H, 13C and 31P NMR spectra (Fig .1) [26].
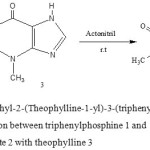 |
Figure 1: Synthesise of dimethyl-2-(Theophylline-1-yl)-3-(triphenylphosphoranylidene) butandioate 4 from the reaction between triphenylphosphine 1 and dimethylacetylendicarboxylate 2 with theophylline 3 |
The adjacent carbonyl group along with the three rotations around the restricted rotational bonds of the synthesized compunds is strongly conjugated in the Z-4 and E-4 rotational isomers (Fig. 2).
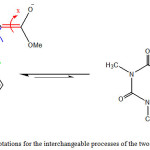 |
Figure 2: Three possible rotations for the interchangeable processes of the two isomers (Z– and E-) in ylide 4 Click here to View figure |
The 1H NMR spectra of the ylide 4 showed two doublets for methine proton (H–C–C=P) and methyl groups δ = 5.493-5.510 and 3.69-3.72 ppm, for the minor and major geometrical isomers, respectively. All interchangeable process of rotational isomers for ylide 4 involving isomers I, II, III, IV, V and VI are shown in Fig. 3
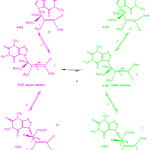 |
Figure 3: The restricted rotational processes for ylide 4
|
Dynamic Effects for the Z-4 and E-4 Rotational Isomers Around the Carbon–Carbon Double Bond (Fig. 3, A, I and II)
The 1H NMR spectrum for the 4-Z and the 4-E (Fig. 3, a, I and II) at º0C (273.15 K) exgibited two sharp doublets for the methine proton (H–C–C=P, 3JPH) in acetone. This spectrum is broadened at 20ºC (293.15 K). The resonance coalescence occurred at near 26 ºC (299.15 K) that they belong to rotational process around carbon–carbon double bond. The methoxy proton resonance coalescence is accrued at near 32 ºC (305.15 K). The coalescence temperature (Tc) and isotropic chemical shift (∆ν) is ibtained for the maximum peak separation in the low-temperature limit [27]. Moreover, parameters and kinetic parameters are calculated by the classic method and tabulated[1] in Table 1.
Table 1: The activation parameters forylide 4 around the carbon-carbon double bound (Entry a, Fig. 3)
|
Tc |
δ |
∆ν |
kc |
∆G‡ |
∆H‡ |
∆S‡ |
Ea |
|
K |
ppm |
Hz |
s-1 |
kcalmol-1 |
kcalmol-1 |
calmol-1K-1 |
kcalmol-1 |
|
299.15 |
5.493-5.510 |
6.8 |
15.1 |
15.9 |
|||
|
305.15 |
3.699-3.729 |
12.01 |
26.66 |
15.88 |
16.58 |
2.28 |
17.17 |
For completing analysis and reducing the errors, the determining of activation parameters are repeated by Eyring and Arrhenius methods. With using the average value of ∆G‡ in various temperature (Table 2), ln(k/T) and lnk can be drawed according to the Eyring[1] and Arrhenius[2] equations [27]. The Eyring and Arrhenius plots are shown in Figs. 4A and 5, respectively. In addition, a different linearized from of Eyring equation has been employed to draw Fig. 4B for determination of ∆H‡ (activation enthalpy) and ∆S‡ (activation energy) [28-30]:
Tln(k/T) = T(23.76 + ∆S‡/R – ∆H‡/RT)
By using some correlation reported previously by researchers, the standard errors of the Eyring and Arrehnius equations are also calculated[3] [28-30]. The values of activation energy and activation parameters obtained by three methods are reported in Table 3.
Table 2. Values of ∆G‡, lnk/T and lnk for rotation around carbon-carbon double bond (Entry a, Fig. 3)
| Tc | 1/T | ∆δ | ∆ ν | ∆G‡ | Lnk/T | Lnk | T×Lnk/T |
| K | ppm | Hz | kcal/mol | ||||
| 263.15 | 0.00380 | 5.479-5.494 | 6.00 | 14.05 | -4.41 | 1.16 | -1159.97 |
| 273.15 | 0.00366 | 5.493-5.510 | 6.80 | 14.51 | -3.38 | 2.23 | -922.154 |
| 283.15 | 0.00353 | 5.508-5.529 | 8.40 | 14.93 | 2.42 | 3.23 | -684.656 |
| 293.15 | 0.00341 | 5.524-5.545 | 8.40 | 15.45 | -1.52 | 4.15 | -477.053 |
: ∆G‡ = aT{9.972 + log(Tc/∆ν) where a = 4.575 × 10-3 for unites of kcal/mol, at the coalescence temperaturekc= πΔυ/√2, ∆H‡ =-R× 2.303 ∆[log(kc/Tc)]/∆[1/Tc], ∆S‡ = (∆H‡ – ∆G‡)/T, Ea = ∆H‡ + nRT
: ln(k/T)=23.76+ ∆S‡/R – ∆H‡/RT,slope = – ∆H‡/R andintercept point= ∆S‡/R +23.76, ∆G‡ = ∆H‡ – T∆S‡, Ea = ∆H‡ + RT
: lnk = lnA – (Ea/R)(1/T), slope = -Ea/R, ∆H‡ = Ea- RT
: σ(∆S‡) = (1/Tav)σ(∆H‡) where Tav= is the center of the temperature range studied, σ(∆S‡) = σ(∆H‡×0.0034 K-1)
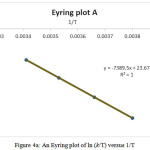 |
Figure 4a: An Eyring plot of ln (k/T) versus 1/T Click here to View figure |
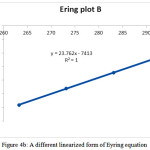 |
Figure 4b: A different linearized form of Eyring equation |
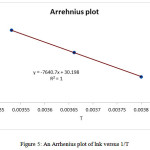 |
Figure 5: An Arrhenius plot of lnk versus 1/T |
Table 3: A comparing between the results obtained by the three methods for the rotation around the carbon-carbondouble bond in the isomer Z-4 and E-4 [entry a]
|
Methods |
∆G‡ (kcalmol-1) |
∆H‡ (kcalmol-1) ∆S‡( calmol-1K-1) |
Ea (kcalmol-1) |
||
|
Classic |
15.9 |
16.58 |
2.28 |
17.17 |
|
|
Eyring(A) |
14.73 |
14.68±0.033 |
-0.18±0.00011 |
15.27±0.033 |
|
|
Eyring(B) |
14.73 |
14.73±0.033 |
0.004 ±0.00011 |
15.32 ±0.033 |
|
|
Arrhenius |
14.64 |
14.59±0.033 |
-0. 18±0.00011 |
15.18 ±0.033 |
|
An evaluation of the results obtained (Table 3) by using three methods shows the results from the Eyring and Arrhenius are consistent, but are different from the classic method.
Dynamic Effect (Fig. 3, B, I and III) for the Carbon–Carbon Single Bond and the Nitrogen–Carbon (N -C-CH) Bond (Fig. 3, C, I and IV) of the Z Isomer
With decreasing temperature lower than -30 ºC, a singlet from methyl proton begin to broad spectrum for the 4–Z isomer. The resonance coalescence can be observed at -69ºC (204.15 K) and the spectrum for the 4–Z isomer (Fig. 3, b, I and III) shows a resonance arising from (3.177-3.194 ppm) in acetone-d6 around the carbon–carbon single bond. Another resonance coalescence observed at near -60ºC (213.15 K) is belong to the methine proton (H–C–C=P, 3JPH) ). For this process, the activation parameters and kinetic parameters is calcualted by using the classic method and is shown in Table 4.
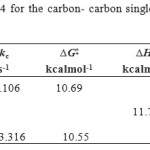 |
Table 4: Activation parameters of ylide 4 for the carbon- carbon single bond in the Z-4 isomers, (I, III) by the classic method Click here to View table |
Also, in lower temperature than -10ºC (8.298 ppm) , a singlet from proton of nitrogen in ring five-membered (N=CH ) begin to broad at -50ºC. The resonance coalescence occurs at approximately -30ºC (243.15 K) and then the 1H NMR spectrum (Fig. 3, c, I and IV) shows a resonance arising from (8.336-8.345 ppm) around the carbon–nitrogen single bond in acetone-d6. Another resonance coalescence is accrued at near -26ºC (247.15 K) is belong to the methine proton (H–C–C=P, 3JPH) ) for the restricted rotational process around nitrogen–carbon single bond. The activation parameters and kinetic parameters are calculated by the classic method and is shown in Table 5.
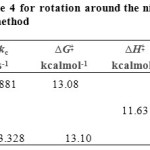 |
Table 5: Activation parameters of ylide 4 for rotation around the nitrogen- carbon single bond in the Z-4 isomers, (I, V) obatiend by the classic method Click here to View table |
Dynamic Effect (Fig. 3, D, II And V) for the Carbon-Carbon Single Bond and the 4-E Rotational Isomers as A Result of Restricted Rotational Processes (Fig. 3, E, II and VI) Around the Nitrogen-Carbon Single Bond
With decreasing temperature in lower than -30ºC, two singlet from methoxy protons beginto broad at -50ºC. The resonance coalescence is observed at -65 ºC (20815 K). Then the 1H NMR spectrum for the 4–E isomer (Fig. 3, d, II and V) shows a resonance arising from (3.685-3.743 ppm) around the carbon–nitrogen simple bond in acetone-d6. Another resonance coalescence is obsreved at near -68ºC (205.15 K) is belong to the methine proton (H–C–C=P, 3JPH) ) for rotational process around carbon–nitrojen single bond. The activation and kinetic parameters are calculated by the classic method and are reported in Table 6.
Table 6: The activation parameters derived from dynamic reviewylide 4 around the carbon-carbon single bound (Entry d, Fig. 3) according to classic method
| Tc | δ | ∆ν | kc | ∆G‡ | ∆H‡ | ∆S‡ | Ea |
| K | ppm | Hz | s-1 | kcalmol-1 | kcalmol-1 | calmol-1K-1 | kcalmol-1 |
| 208.15 | 3.685-3.743 | 23.21 | 51.54 | 10.4 | |||
| 11.55 | 5.54 | 11.56 | |||||
| 205.15 | 5.317-5.355 | 15.208 | 33.767 | 10.39 |
The Eyring plot and Arrhenius plot are shown in Figs. 5 and 6 and also the activation and kinetic parameters are calculated by using the classic method and also are presented in Table 7.
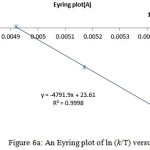 |
Figure 6a: An Eyring plot of ln (k/T) versus 1 |
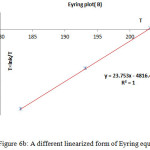 |
Figure 6b: A different linearized form of Eyring equation |
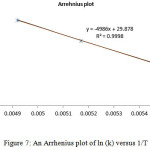 |
Figure 7: An Arrhenius plot of ln (k) versus 1/T
|
Table 7: Comparing the obtained results from the two methods (Eyring and Arrhenius) for the restricted rotation around the carbon-carbon single bond in the isomers E-4 [entry d]
| Methods | ∆G‡(kcalmol-1) | ∆H‡(kcalmol-1) | ∆S‡(kcalmol-1K-1) | a(kcalmol-1) |
| Classic | 10.4 | 11.55 | 5.54 | 11.96 |
| Eyring(A) | 9.58 | 9.52± 0.1 | -0.30 ± 0.00034 | 9.93 ±0.1 |
| Eyring(B) | 9.57 | 9.57± 0.1 | -0.014 ± 0.00034 | 9.98 ±0.1 |
| Arrhenius | 9.56 | 9.49± 0.1 | -0. 30± 0.00034 | 9.9.91 ±0.1 |
The results from Eyring and Arrhenius plots are consistent togetehr more than the classic method (Table 7). Moreover, when temperature is reduced down lower than -10 ºC a singlet from proton of nitrogen in ring five-membered (N=CH ) begin to broad at -25ºC.The resonance coalescence occurs at -29 ºC (244.15 K). Then the 1H NMR spectrum for the 4–E isomer (Fig. 3, e, II and VI) shows a resonance arising from (8.393-8.404 ppm) around the carbon–nitrogen simple bond in acetone-d6. Another resonance coalescence is accrued at near -27ºC (246.15 K) in relation to the methine proton (H–C–C=P, 3JPH) ) which is relevant to restricted rotational process around carbon–nitrojen single bond. For this process, the activation parameters and kinetic parameters obtained using the classic method is tabulated in Table 8. Because of lone pair inversion of nitrogen atom in heteroatom five-membered ring, rotation around the carbon-carbon single bond requires a barrier rotational energy more than the carbon-carbon single bond (comparison between Ea and ∆G‡ in Table 8).
Table 8: Activation parameters of ylide 4 for rotation around the nitrogen-carbon single bond in the E-4 isomers, (II ,VI) by the classic method
|
Tc |
δ |
∆ν |
kc |
∆G‡ |
∆H‡ |
∆S‡ |
Ea |
|
K |
ppm |
Hz |
s-1 |
kcalmol-1 |
kcalmol-1 |
calmol-1K-1 |
kcalmol-1 |
|
244.15 |
8.393-8.404 |
4.4 |
9.77 |
13.09 |
|||
|
9.49 |
-14.7 |
9.94 |
|||||
|
246.15 |
5.408-5.421 |
5.202 |
11.55 |
13.2 |
Conclusion
The three most common methods for calculating the activation parameters of the restricted rotational isomers are used. Results obtained from the Eyring and Arrhenius methods were consistent, however, were different with the classic method for the carbon-carbon double bond. Results from Eyring and Arrhenius plots agree more than the classic method for carbon-carbon single bond in E isomer. The activation parameters were calculated using the classic method (single point) for nitrogen–carbon in E and Z isomer and carbon-carbon Z isomer. The Eyring and Arrhenius method did not use in these rotation because required data were not available. Because of the strong conjugation with the adjacent carbonyl group, the value of activation barrier energy (∆G‡) of the restricted rotation around the partial carbon-carbon double bond (MeO2C=CPPh3) was larger than the nitrogen-carbon (N-C-CH) and carbon-carbon (MeO2CH-C-C=PPh3) single bonds. As a case study, because of the lone pair inversion of nitrogen atom in heteroatom -membered ring, rotation around the carbon-nitrogen single bond needs a barrier rotational energy more than the carbon-carbon single bond. Rotation around the carbon-carbon double bond ∆G‡ has a more positive value. So high value of ∆G‡ indicates the rate of this process is slow at room temperature. At higher temperature it is fast and spontaneous. Rotation around the carbon-carbon single bond ∆G‡ has low value (less position value), so rotation around the carbon-carbon single bond is a fast process and spontaneous at room temperature, observation of two conformers (I, II or III and IV) on the basis of this rotation at low temperature is a good evidence for the process that is not spontaneous under this condition.
Acknowledgement
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
References
- Tawkir, M.; Iqbal, S. A.; Krishan, B.; Zaafarany, I. Orient. J. Chem. 2011, 27(2), 603-609.
- Hazeri, N.; Habibi-Khorassani, S. M.; Maghsoodlou, M. T.; Marandi, G.; Nassiri, M.; Shahzadeh, G. J. Chem. Res. 2006, 3, 215-217.
CrossRef - Maghsoodlou, M. T.; Habibi-Khorassani, S. M.; Hazeri, N.; Nassiri, M.; Kakaei, R.; Marandi, G. Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181(3), 553-560.
CrossRef - Maryanoff, B. E.; Rietz, A. B. Chem. Rev. 1989, 89(4), 863-927.
CrossRef - An-Hu, L.; Li-Xin, D.; Aggrwal, V. Chem. Rev. 1997, 97(6), 2341-2372.
CrossRef - Kolodiazhnyi, O. I. Russ. Chem. Rev. 1997, 66(3), 225-254.
CrossRef - Pietrusiewiz, K. M.; Zablocka, M. Chem. Rev. 1994, 94(5), 1375-1411.
CrossRef - Profire, L.; Şunel, V.; Lupascui, D.; Baican, M. C.; Bibire, N.; Vasile, C. Farmacia. 2010, 58(2),170-176.
- Barnes, P. J.; Pauwels, R. A. Eur. Respir. J. 1994, 3, 579–591.
CrossRef - Kawayama, T.; Hoshino, T.; Ichiki, M.; Tsuda, T.; Kinoshita, M.; Takata, S.; Koga, T.; Iwanaga, T.; Aizawa, H. Int. J. Chron. Obstruct. Pulmon. Dis. 2008, 3(1), 137-148.
- Korzycka, L.; Górska, D. J. Pharm. Pharmacol. 2008, 60(5), 637-645.
CrossRef - Majumdar, S.; Sloan, K. B. Int. J. Pharm. 2007, 33, 64-71.
CrossRef - Marwick, J. A.; Ito, K.; Adcoc, I. M.; Kirkham, P.A. Expert Opin. Ther.Targets. 2007, 11(6), 745-755.
CrossRef - Buck, M. L.; D. Marcia.; Pharm. 2008, 14, 6-20.
- Finney, M. JB.; Karlson, J. A.; Persson, C. J. Pharmacol. 1985, 85, 29-36.
- Guillot, C.; Fornaris, M.; Badger, M.; Orehek, J. J. Allergy. ClinImmunol. 1984, 74(5), 713-718.
CrossRef - Finnerty, J. P.; Lee, C.; Wilson, S.; Madden, J.; Djukanovic, R.; Holgate, S.T.; Eur. Respir. J. 1996, 9, 1672-1677.
CrossRef - Auchampach, J. A.; Kreckler, L. M.; Wan, T. C.; Maas, J. E.; Hoeven, D. V.; Gizewski, E. Narayanan, J.; Maas, G. E. J. Pharmacol. Exp. Ther. 2009, 329(1), 2-13.
CrossRef - Srdjenovic, B.; Djordjevic-Milicn, V.; Grujic, R.; Injac, Z. J. Chromatogra. Sci. 2008, 46(2), 144-149.
CrossRef - Anet, F. A. L.; Anet, R.; Cotton, F. A.; Jackman, L.M. Eds., Academic Press: New York, 1979.
- Mofarrah, E.; Habibi-Khorassani, S. M.; Maghsoodlou, M.T.; Shahraki, M. Indian J. Chem. Sect B. 2015, 54(B), 1528-1534.
- Mofarrah, E.; Habibi-Khorassani, S. M.; Maghsoodlou, M. T.; Shahraki M. Appl. Magn. Reson. 2015, 46 (10), 1179-1188.
CrossRef - Habibi-Khorassani, S.M.; Maghsoodlou, M. T.; Ebrahimi, A.; Mohammadi, M.; Shahraki, M.; Aghdaei, E. J. Phys. Org. Chem. 2012, 25(12), 1328-1335.
CrossRef - Habibi-Khorassani, S. M.; Shahraki, M.; Maghsoodlou, M.T.; Erfani, S. Spectrochim. Acta, Part A. 2015, 145, 410–416.
CrossRef - Habibi-Khorassani, S. M.; Shahraki, M.; Maghsoodlou, M. T.; Mofarrah, EI.; Mofarrah, EL.; Mousavi, S. R. Biomed. Pharmacol. J. 2015, 8(2), 565-572.
CrossRef - Aghdaei, E.; Habibi-Khorassani, S. M.; Shahraki, M. Orient. J. Chem. 2015, 31(4), 2427-2433.
CrossRef - Zimmer, K. D.; Shoemaker, R.; Rumi-Ski R. R. Inorganic Chemical Acta. 2006, 359(5), 1478-1484.
CrossRef - Lente, G.; Fabian, I.; Poe, A. J.; New J. Chem. 2005, 29(6), 759-760.
CrossRef - Espenson, J. H. McGraw-Hill, New York, 1995, 2,158.
- Poe, A. J.; ed. Twigg, M.V. Plenum Press. 1994, 8,10, 220.

This work is licensed under a Creative Commons Attribution 4.0 International License.









