Synthesis of Benzo[G]Quinoxaline-5,10-Dione Based Pyridine Derivatives and their Antimycobacterial Activity
Shiv Kumar1, Nitin Kumar2 and Sushma Drabu3
1Faculty of Pharmacy, Uttrakhand Technical University, Dehradun-248007, India.
2Oxford College of Pharmacy, Masoori Industrial Area, U.P.S.I.D.C., M.G. Road Ghaziabad-201001, India.
3Maharaja Surajmal Institute of Pharmacy, Janak Puri, New Delhi-110058, India.
Corresponding Author E-mail: shiv_hamdard@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/330230
Article Received on : March 25, 2017
Article Accepted on : January 11, 2017
Thirteen new compounds belonging to series 2-amino-6-(5,10-dioxo-2,3-diphenyl-5,10-dihydrobenzo[g]quinoxalin-7-yl)-4-(substituted)phenylpyridine-3-carbonitrile (6a-m) were synthesized by multistep synthetic scheme. The newly synthesized compounds were screened for their antimycobacterial activity by L.J. Slope (Conventional) Method. Compound 6h with 4-N(CH3)2 group at phenyl ring of above mentioned position has been reported as most active antimycobacterial compound and compound 6k with 4-CH3 substitution at phenyl above mentioned position has been reported as the least active antimycobacterial compound.
KEYWORDS:Quinoxaline; Benzo[g]quinoxaline-5,10-dione; Pyridine; Antimycobacterial
Download this article as:| Copy the following to cite this article: Kumar S, Kumar N, Drabu S. Synthesis of Benzo[G]Quinoxaline-5,10-Dione Based Pyridine Derivatives and their Antimycobacterial Activity. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Kumar S, Kumar N, Drabu S. Synthesis of Benzo[G]Quinoxaline-5,10-Dione Based Pyridine Derivatives and their Antimycobacterial Activity. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31248 |
Introduction
Quinoxaline and pyridine derivatives constitute the important part of various antibiotics viz. hinomycin, levomycin and actinoleutin that inhibit the growth various bacteria and are found to be cytotoxic against various transplantable tumours1-7. Quinoxaline derivatives also exhibited a wide range of biological activities viz. antifungal8-10, antibacterial11-12, antitubercular8-9,12-15.
Tuberculosis (TB) is a major health problem worldwide now-a-days. In year 2014, there were an estimated 9.6 million new TB cases were reported and 1.5 million deaths were caused due to TB worldwide16. Quinoxaline ring is also a an important part of antileprotic drug clofazimine (CZM) which is also widely indicated for treatment of multidrug resistant tuberculosis. In Mycobacterium tuberculosis, CZM is reduced by NADH-dehydrogenase (NDH-2) to release reactive oxygen17 and CZM also act as competitor with menaquinone (MK-4), which acts as a key factor for its reduction by NDH-218.First line antitubercular drug isoniazid (INH) and second line antitubercular drug ethionamide (Etm) are derivatives of six membered nitrogen containing pyridine ring. Isoniazid and Ethionamide, after bioactivation by mycobacterial catalase-peroxidase enzyme complex, inhibit synthesis of mycolic acids19-23, that are important components of maycobacterial cell wall.
Experimental
Chemistry
Melting points were measured in open capillary tubes and are uncorrected. All the Fourier-Transform Infra Red (FT-IR) spectra were recorded on Shimdazu FT-8400 Spectrophotometer using KBr pallets. The 1H- NMR spectra were recorded on Bruker-Spectrospin DCX NMR spectrometer using DMSO-d6 and CDCl3 as solvents and tetramethylsilane (TMS) as an internal standard (Chemical shifts expressed in δ/ppm The purity of the compounds was checked by thin layer chromatography (TLC) on Merck Silica Gel 60F254 precoated sheets using Toluene : Ethylacetate : Formic acid (5:4:1) solution mobile phase.
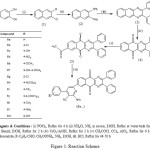 |
Figure 1: Reaction Scheme
|
Synthesis of 2,3-Dichloronaphthalene
2,3-dihydroxynaphthalene (5 gm) was dissolved in phosphorusoxychloride (90 mL) in parts at 0o-5oC with occasionally stirring and refluxed for about 4 h till the appearance of clear dark pinkish solution. This solution was cooled and poured into ice-cold water. A dark pinkish solid appeared and was filtere, dried and recrystallized with dimethylformaide (DMF). Pinkish crystalline solid; Yield 85%; Rf 0.72; mp 185oC. IR (vmax, KBr, cm-1): 756 (ortho-disubstituted phenyl ring), 856 (meta-disubstituted phenyl ring), 1160 (aryl C-Cl), 1384 (aromatic C=C); 1H-NMR (CDCl3 300 MHz) δ : 8.16-8.20 (q, J=5-6 Hz, A2B2 pattern, 2H, Ar-H), 7.74-7.79 (m, J=4.5-6 Hz, 2H, Ar-H), 7.50-7.53 (m, J=1-4 Hz, 2H, Ar-H)
Synthesis of 2,3-Diaminonaphthalene
Compound 1 (2 gm) was dissolved in absolute ethanol. Ammonium chloride (1.5 gm), dil. HCl (in catalytic amount) and excess of strong ammonium solution were added to it and refluxed for 6 h on water bath till appearing of permanent dark greenish colour. After completion of reaction, solution was cooled and kept in deep freezer for overnight. Greenish crystals were filtered, dried and recrystallized with methanol. Dark greenish crystals; Yield 75%; Rf 0.89, Rf 0.82; mp 150oC; IR (vmax, KBr, cm-1): 758, 854, 1473 (aromatic =C-N), 1500 (aromatic C=C), 1681 (N-H), 3049 (N-H); 1H-NMR (CDCl3 500 MHz): 7.45-7.46 (dd, J=3.5-4 Hz, 2H, Ar-H), 7.12-7.14 (dd, J=3-3.5 Hz, 2H, Ar-H), 7.09 (s, Ar-H, 2H), 7.05 (s, Ar-NH2,4H).
2,3-Diphenylbenzo[g]Quinoxaline
Equimolar quantities of compound 2 (0.001 mol) and benzil (0.001 mol) were dissolved in ethanol (30 mL) and refluxed for 2 h. After completion of reaction, reaction mixture was cooled, kept for overnight, yellow crystalline solid was filtered, dried and recrystallized with ethanol : methanol (1:1) mixture. Yellow crystalline solid; Yield 72%; Rf 0.83; mp 170oC; IR (vmax,KBr, cm-1) : 725, 785, 820, 875, 1475, 1510 (aromatic C=C), 1660 (quinoxaline C=N); 1H-NMR (DMSO-d6 500 MHz) δ: 8.73-8.77 (m, J=2.5-4 Hz, 10H, Ar-H), 8.28 (s, 2H, Ar-H), 8.03-8.07 (m, 4H, J=2.5-4 Hz, Ar-H).
Synthesis of 2,3-Diphenylbenzo[g]Quinoxaline-5,10-Dione
Compound 3 (1.2 gm) was dissolved in glacial acetic acid (30 mL) and chromium trioxide (1.1 gm) in 12 mL of glacial acetic acid : water (1:1) solution was added to it. This solution was refluxed at 80oC for 2 h and then poured into ice-chilled water (1500 mL). Resulting solid was filtered, dried, recrystallized with methanol. White solid; Yield 65%; Rf 0.78; mp 162oC; IR (vmax, KBr, cm-1): 718, 795, 875, 1450, 1594 (>C=O, cyclic, conjugated), 1676; 1H-NMR (CDCl3 500 MHz) δ: 8.13-8.15 (d, 2H, J=8.5 Hz, Ar-H), 7.85-7.88 (t, J=2.5-10.5 Hz, 4H, Ar-H), 7.81-7.82 (d, J=8 Hz, 2H, Ar-H), 7.50-7.53 (t, J=7.5 Hz, 6H, Ar-H); Elemental Anal. Found (Calcd.) for C24H14N2O2: Found: C, 79.42 (79.55); H 3.91 (3.89); N 7.68 (7.73); O 8.95 (8.83).
Synthesis of 7-Acetyl-2,3-diphenylbenzo[g]Quinoxaline-5,10-dione
Anhydrous aluminium trichloride (1 gm) was dissolved in carbon tetrachloride (30 mL) and acetyl chloride (2 mL) was added to it under cold conditions (0o-5oC). Compound 4 (120 mg) was added and stirred at room temperature for 6 h. Yellow crystalline solid was filtered, dried and recrystallized with chloroform. Yellow crystalline solid; Yield 48%; Rf 0.74; mp 225oC; IR (vmax, KBr, cm-1): 725, 775, 810, 875, 1450, 1500, 1593 (>C=O, cyclic, conjugated), 1672 (C=N, quinoxaline), 1974 (>C=O, aliphatic), 3063 (C-H, sp3, CH3); 1H-NMR (CDCl3 500 MHz) δ: 7.97-7.98 (d, J=8 Hz, 4H, Ar-H), 7.65-7.68 (t, J=7.5 Hz, 3H, Ar-H), 7.50-7.53 (t, 6H, J=7.5-8 Hz, Ar-H), 7.26 (s, 3H, CH3CO); MS-ESI: 404.14 (m/z, M+); Elemental Anal. Found (Calcd.) for C26H16N2O3 : C 77.23 (77.22); H 3.98 (3.99); N 6.97 (6.93); O 11.82 (11.87).
Synthesis of 2-Amino-6-(5,10-Dioxo-2,3-Diphenyl-5,10-Dihydrobenzo[g]Quinoxalin-7-yl)-4-(Substituted) Phenylpyridine-3-Carbonitrile
Equimolar quantities of compound 5 (0.01 mol), appropriate benzaldehyde (0.01 mol), ammonium acetate (2 gm) and malononitrile (0.01 mol) and were dissolved in ethanol (30 mL) and was refluxed under anhydrous conditions for 48-50 h till the permanent appearance of crystalline solid. Solid was filtered, dried and recrystallized with chloroform.
2-Amino-6-(5,10-Dioxo-2,3-Diphenyl-5,10-Dihydrobenzo[g]Quinoxalin-7-yl)-4-Phenyl Pyridine-3-Carbonitrile
Yellow crystalline solid; Yield 74%; Rf 0.84; mp 230oC; IR (vmax, KBr, cm-1): 719, 756, 820, 875, 1296 (C=N, pyridine), 1384 (C-N), 1608 (>C=O, cyclic, conjugated), 1642 (N-H, primary amine, bend), 1697 (C=N, quinoxaline), 2358 (C-N, nitrile), 3417 (N-H, primary amine, str.); 1H-NMR (CDCl3 500 MHz) δ: 12.47 (s, 1H, Ar-H), 11.91 (s, 2H, Ar-H), 11.73 (bs, 2H, Ar-NH2), 9.03-9.04 (d, J=5 Hz, 1H, Ar-H), 8.01-8.02 (d, 2H, J=8 Hz, Ar-H), 7.75-7.79 (t, J=8-12 Hz, 3H, Ar-H), 7.59-7.63 (t, J=8.5-9.5 Hz, 4H, Ar-H), 7.12-7.30 (m, J=7.5-14.5 Hz, 6H, Ar-H); MS-ESI: 555.05 (m/z, M+); Elemental Anal. Found (Calcd.)for C36H21N5O2 : C, 77.80 (77.83); H, 3.82 (3.81); N, 12.60 (12.61); O, 5.76 (5.76).
2-Amino-(4-Chlorophenyl)-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[G]Quinoxalin-7-yl}Pyridine-3-Carbonitrile
Yellow crystalline solid; Yield 80%; Rf 0.80; mp 232oC; IR (vmax, KBr, cm-1): 723, 760, 819, 875, 1162 (aryl C-Cl), 1284, 1383, 1592, 1645, 1697, 2363, 3404; 1H-NMR (DMSO-d6 500 MHz) δ: 8.73-8.77 (m, J=2.5-4 Hz, 10H, Ar-H), 8.03-8.07 (m, J=3.0-3.5 Hz, 4H, Ar-H), 7.70-7.78 (q, J=7.5-15 Hz, 1H, Ar-H), 7.36-7.38 (d, J=8.5 Hz, 1H, Ar-H), 7.28-7.30 (d, J=7.5 Hz, 1H, Ar-H), 7.16-7.19 (t, J=8-7.5 Hz, 1H, Ar-H), 6.94-6.96 (d, J=8 Hz, 2H, Ar-NH2); MS-ESI: 589.08 (m/z, M+); Elemental Anal. Found (Calcd.) for C36H20ClN5O2 : C, 73.27 (73.28); H, 3.43 (3.42); Cl, 5.98 (6.01); N, 11.89 (11.87); O, 5.42 (5.42).
2-Amino-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}-4-(4-Hydroxyphenyl) Pyridine-3-Carbonitrile
Yellow crystalline solid; Yield 72%; Rf 0.78; mp 250oC; IR (vmax, KBr, cm-1) : 718, 765, 827, 895, 1256 (phenolic C-O phenolic), 1351, 1402, 1596, 1679, 1683, 2358, 3146, 3367 (O-H phenolic); 1H-NMR (DMSO-d6 500 MHz) δ: 9.12 (s, 1H, Ar-H), 8.72-8.76 (m, J=2.5-4 Hz, 10H, Ar-H), 8.56-8.58 (d, J=9 Hz, 1H, Ar-H), 8.48-8.49 (d, J=9 Hz, 1H, Ar-H), 7.99-8.11 (t, J=3-5 Hz, 1H, Ar-H), 7.68-7.72 (dd, J=9 Hz, 2H, Ar-H), 7.44-7.49 (dd, J=9 Hz, 2H, Ar-H), 6.98 (s, 2H, Ar-NH2), 6.86-6.88 (t, J=3-4 Hz, 1H, Ar-OH); MS-ESI: 571.11 (m/z, M+); Elemental Anal. Found (Calcd.) for C36H21N5O3 : C, 75.62 (75.65); H, 3.69 (3.70); N, 12.27 (12.25); O, 8.39 (8.40).
2-Amino-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}-4-(4-Nitrophenyl) Pyridine-3-Carbonitrile
Yellow crystalline solid; Yield 68%; mp 210oC; Rf 0.80; IR (vmax, KBr, cm-1): 720, 758, 811, 854, 1284, 1383 (-NO2, sym.), 1410, 1545 (-NO2, asym.), 1594, 1669, 1684, 2359, 3335; 1H-NMR (DMSO-d6 500 MHz) δ: 9.23-9.27 (dd, J=9.5 Hz, 2H, Ar-H), 9.11-9.11 (d, J=3 Hz, 1H, Ar-H), 8.87-8.92 (dd, J=9 Hz, 2H, Ar-H), 8.82-8.86 (m, J=2.5-5 Hz, 10H, Ar-H), 8.76-8.78 (d, J=11 Hz, 1H, Ar-H), 8.68-8.70 (d, J=11 Hz, 1H, Ar-H), 8.59-8.60 (t, J=3-4.5 Hz, 1H, Ar-H), 7.01 (s, 2H, Ar-NH2); MS-ESI: 600.14 (m/z, M+); Elemental Anal. Found (Calcd.) for C36H20N6O4 : C, 71.98 (71.99); H, 3.34 (3.36); N, 14.02 (13.99); O, 10.65 (10.66).
2-Amino-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}-4-(4-Methoxyphenyl) Pyridine-3-Carbonitrile
White crystalline solid; Yield 74%; Rf 0.78; mp 200oC; IR (vmax, KBr, cm-1): 719, 758, 826, 853, 1130 (C-O-C, sym.), 1253 (C-O-C, asym.), 1340, 1420, 1595, 1645, 1677, 2360, 3315; 1H-NMR (DMSO-d6 500 MHz) δ: 8.73-8.76 (m, J=2.5-4 Hz, 10H, Ar-H), 8.03-8.07 (m, J=3-4 Hz, 4H, Ar-H), 7.74 (s, 1H, Ar-H), 7.67 (s, 1H, Ar-H), 7.44-7.46 (d, J=7.5 Hz, 1H, Ar-H), 7.28-7.30 (d, J=7.5 Hz, 1H, Ar-H), 7.13-7.17 (t, 2H, Ar-NH2), 3.91 (s, 3H, Ar-OCH3); MS-ESI: 585.13 (m/z, M+); Elemental Anal. Found (Calcd.) for C37H23N5O3 : C, 75.90 (75.89); H, 3.95 (3.96); N, 11.97 (11.96); O, (8.18) 8.20.
2-Amino-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}-4-(3-Hydroxy-4 Methoxyphenyl)Pyridine-3-Carbonitrile
Brown crystalline solid; Yield 72%; Rf 0.81; mp 210oC;IR (vmax, KBr, cm-1): 720, 757, 838, 865, 1124 (C-O-C, sym.), 1252 (C-O-C, asym.), 1295 (C-O, phenolic), 1342, 1424 (C-N), 1611, 1660, 1673, 2360, 3326, 3381 (O-H, phenolic); 1H-NMR (CDCl3 500 MHz) δ: 7.80-7.81 (d, J=7 Hz, 10H, Ar-H), 7.96-7.97 (d, J=7.5 Hz, 1H, Ar-OH), 7.09 (s, 2H, Ar-NH2), 7.33-7.36 (t, J=7.5-8 Hz, 3H, Ar-H), 7.48-7.51 (t, J=2.5-7.5 Hz, 2H, Ar-H), 7.63-7.66 (t, J=7-8 Hz, 1H, Ar-H), 7.16-7.20 (t, J=7.5-8.5 Hz, 1H, Ar-H), 3.31 (s, 3H, Ar-OCH3); MS-ESI: 601.16 (m/z, M+); Elemental Anal. Found (Calcd.) for C37H23N5O4 : C, 73.85 (73.87); H, 3.86 (3.85); N, 11.65 (11.64); O, 10.63 (10.64).
2-Amino-4-(2-Chlorophenyl)-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}Pyridine-3-Carbonitrile
White crystalline solid; Yield 66%; Rf 0.72; mp 180oC; IR (vmax, KBr, cm-1): 736, 761, 815, 892, 1164 (aryl C-Cl), 1311, 1363, 1591, 1662, 2356, 3305; 1H-NMR (DMSO-d6 500 MHz) δ: 8.74-8.76 (q, J=2.5-3.5 Hz, 4H, Ar-H), 8.04-8.07 (m, J=3.5-5 Hz, 10H, Ar-H), 7.96 (s, 1H, Ar-H), 7.91-7.93 (t, J=2.5–8.5 Hz, 1H, Ar-H), 7.68-7.71 (d, 2H, Ar-NH2), 7.51-7.53 (d, J=8.5 Hz, 1H, Ar-H), 7.37-7.39 (d, J=8 Hz, 1H, Ar-H); MS-ESI: 589.14 (m/z, M+); Elemental Anal. Found (Calcd.) for C36H20ClN5O2 : C, 73.28 (73.28); H, 3.41 (3.42); Cl, 6.00 (6.01); N, 11.88 (11.87); O, 5.42 (5.42).
2-Amino-4-[4-(Dimethylamino)Phenyl]-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}Pyridine-3-Carbonitrile
Lemon crystalline solid, yield 76%; Rf 0.80; mp 185oC; IR (vmax, KBr, cm-1): 723, 759, 819, 837, 1325, 1411, 1593, 1647, 2360, 2958 (C-H, sp3, CH3), 3282; 1H-NMR (DMSO-d6 500 MHz) δ: 8.73-8.77 (m, J=3-4 Hz, 10H, Ar-H), 8.03-8.07 (m, J=3-4 Hz, 4H, Ar-H), 8.28 (s, 1H, Ar-H), 7.93 (s, 1H, Ar-H), 7.65 (s, 1H, Ar-H), 7.28 (s, 1H, Ar-H), 7.14 (s, 2H, Ar-NH2), 3.49 (s, 6H, N-CH3); MS-ESI: 598.31 (m/z, M+); Elemental Anal. Found (Calcd.) for C38H26N6O2 : C, 76.22 (76.24); H, 4.37 (4.38); N, 14.05 (14.04); O, 5.34 (5.35).
2-Amino-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}-4-[4-(Trifluoromethyl)Phenyl]Pyridine-3-Carbonitrile
Brown crystalline solid; Yield 78%; Rf 0.80; mp 178oC; IR (vmax, KBr, cm-1): 723, 741, 843, 863, 1285 (C-F), 1320, 1420, 1603, 1645, 1675, 2365, 3236; 1H-NMR (DMSO-d6 500 MHz) δ: 8.73-8.77 (m, J=3-4 Hz, 4H, Ar-H), 8.28 (s, 1H, Ar-H), 8.03-8.07 (m, J=3-4.5 Hz, 10H, Ar-H), 7.73-7.84 (d, J=8.5 zHzHHHHz, 1H, Ar-H), 7.35-7.37 (d, J=8 Hz, 1H, Ar-H), 7.76 (s, 1H, Ar-H), 7.02-7.03 (d, J=4.5 Hz, 2H, Ar-NH2); MS-ESI: 623.14 (m/z, M+); Elemental Anal. Found (Calcd.) for C37H20FN5O2 : C, 71.26 (71.27); H, 3.24 (3.23); F, 9.12 (9.14); N, 11.24 (11.23); O, 5.13 (5.13).
2-Amino-4-(3,4-Dimethoxyphenyl)-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}Pyridine-3-Carbonitrile
White crystalline solid; Yield 66%; Rf 0.75; mp 144oC; IR (vmax, KBr, cm-1): 720, 752, 821, 854, 1125 (C-O-C, sym.), 1246 (C-O-C, asym.), 1337, 1418, 1608, 1645, 1682, 2357, 3246; 1H-NMR (DMSO-d6 500 MHz) δ: 8.98 (s, 1H, Ar-H), 8.83-8.94 (m, J=3.5-12 Hz, 10H, Ar-H), 8.11-8.12 (d, J=8 Hz, 1H, Ar-H), 8.01-8.03 (d, J=8 Hz, 1H, Ar-H), 7.92-7.94 (d, J=8.5 Hz, 1H, Ar-H), 7.83 (bs, 2H, Ar-NH2), 7.24-7.26 (d, J=10 Hz, 1H, Ar-H), 7.21-7.31 (d, J=9.5 Hz, 1H, Ar-H), 6.91-6.93(d, J=10 Hz, 1H, Ar-H), 3.38 (s, 6H, Ar-OCH3); MS-ESI: 615.19 (m/z, M+); Elemental Anal. Found (Calcd.) for C38H25N5O4 : C, 74.16 (74.14); H, 4.07 (4.09); N, 11.38 (11.38); O, 10.39 (10.40).
2-Amino-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}-4-(4-Methyl Phenyl)Pyridine-3-Carbonitrile
Yellow crystalline solid; Yield 70%; Rf 0.70; mp 140oC; IR (vmax, KBr, cm-1): 723, 750, 817, 850, 1328, 1406, 1606, 1650, 2358, 2839 (C-H, sp3, CH3), 3209; 1H-NMR (DMSO-d6 500 MHz) δ: 8.88 (s, 1H, Ar-H), 8.56-8.58 (d, J=10 Hz, 1H, Ar-H), 8.48-8.51 (d, J=10.5 Hz, 1H, Ar-H), 8.42-8.43 (t, J=3.5-4 Hz, 1H, Ar-H), 8.11-8.18 (m, J=2.5-7.5 Hz, 10H, Ar-H), 7.82-7.99 (m, 4H, Ar-H), 6.88 (s, 2H, Ar-NH2), 3.58-3.59 (t, J=1.5-5 Hz, 3H, CH3); MS-ESI: 569.17 (m/z, M+); Elemental Anal. Found (Calcd.) for C37H23N5O2 : C, 78.03 (78.02); H, 4.06 (4.07); N, 12.30 (12.29); O, 5.60 (5.62).
2-Amino-4-(4-Aminophenyl)-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}Pyridine-3-Carbonitrile
Yellow crystalline solid; Yield 74%; Rf 0.84; mp 180oC; IR (vmax, KBr, cm-1): 725, 750, 820, 860, 1325, 1415, 1650 (N-H, primary amine, bend), 1658, 2358, 3240; 1H-NMR (DMSO-d6 500 MHz) δ: 7.24-7.26 (q, J=2.5-3 Hz, 10H, Ar-H), 7.01 (s, 1H, Ar-H), 6.92-6.93 (t, J=2.5-3 Hz, 4H, Ar-H), 6.89-6.91 (d, J=10 Hz, 2H, Ar-H), 6.85 (s, 1H, Ar-H), 6.06 (bs, 4H, Ar-NH2); MS-ESI: 570.18 (m/z, M+); Elemental Anal. Found (Calcd.) for C36H22N6O2 : C, 75.79 (75.78); H, 3.90 (3.89); N, 14.73 (14.73); O, 5.58 (5.61).
2-Amino-6-{5,10-Dioxo-2,3-Diphenyl-5H,10H-Benzo[g]Quinoxalin-7-yl}-4-(2-Hydroxyphenyl)pyridine-3-Carbonitrile
Yellow crystalline solid; Yield 72%; Rf 0.86; mp 220oC; IR (vmax, KBr, cm-1): 725, 760, 820, 870, 1280, 1311 (C-O, phenolic), 1382, 1595, 1650, 2358, 3380 (O-H, phenolic), 3406; 1H-NMR (DMSO-d6 500 MHz) δ: 8.78 (bs, 1H, Ar-H), 8.64-8.66 (d, J=10 Hz, 1H, Ar-H), 8.56-8.59 (t, J=4-10 Hz, 1H, Ar-H), 8.48-8.50 (t, J=4-5 Hz, 1H, Ar-H), 8.22-8.31 (m, J=2.5-8.5 Hz, 10H, Ar-H), 7.12-7.18 (m, J=2.5-10 Hz, 4H, Ar-H), 6.94 (bs, 2H, Ar-NH2), 6.86-6.87 (d, J=3 Hz, 1H, Ar-OH); MS-ESI: 571.14 (m/z, M+); Elemental Anal. Found (Calcd.) for C36H21N5O3 : C, 75.64 (75.65); H, 3.69 (3.70); N, 12.27 (12.25); O, 8.39 (8.40).
In-vitro Antimycobacterial Screening
Ten compounds 6a, 6b, 6c, 6d, 6f, 6g, 6h, 6i, 6j and 6k were submitted for their antimycobacterial activity against Mycobacterium tuberculosis H37Rv (MTCC-200) by L.J. Slope (Conventional) Method24-25. Each compound was diluted to 2000 μg/mL concentration (as a stock solution). Inoculum Size for Mycobacterium tuberculosis was adjusted to 1 mg/mL. L.J. Medium was used as nutrient medium. Isoniazid (0.20 µg/mL) and rifampicin (0.25 µg/mL) were used as standard drug.
Primary Screen
In primary screening, the compounds were diluted to 500 µg/mL, 250 µg/mL and 125 µg/mL concentrations. Compounds found active in this primary screening were further tested in a second set of dilution against Mycobacterium tuberculosis.
Secondary Screen
The compounds found active in primary screening were further diluted to 100 µg/mL, 50 µg/mL, 25 µg/mL, 12.5 µg/mL, 6.250 µg/mL, 3.125 µg/mL and 1.5625 µg/mL concentrations.
Reading Result
The highest dilution showing at least 99% inhibition was considered as Minimum Inhibitory Concentration. The inoculum size of test should contain 108 organism/mL.
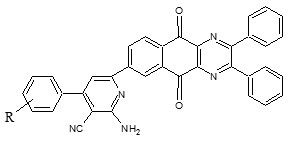
Table 1. Antimycobacterial activity of synthesized compounds against M. tuberculosis H37Rv by L. J. Slope Method.
|
Compound |
R |
MIC (μg/mL) |
|
6a |
H |
125 |
|
6b |
4-Cl |
62.5 |
|
6c |
4-OH |
100 |
|
6d |
4-NO2 |
500 |
|
6e |
4-OCH3 |
— |
|
6f |
3-OH-4-OCH3 |
500 |
|
6g |
2-Cl |
62.5 |
|
6h |
4-N(CH3)2 |
50 |
|
6i |
4-CF3 |
100 |
|
6j |
3,4-(OCH3)2 |
250 |
|
6k |
4-CH3 |
500 |
|
6l |
4-NH2 |
— |
|
6m |
2-OH |
— |
|
Rifampicin |
— |
0.25 |
|
Isoniazid |
— |
0.20 |
Result and Discussion
Chemistry
Thirteen new compounds belonging to series 2-amino-6-(5,10-dioxo-2,3-diphenyl-5,10-dihydrobenzo[g]quinoxalin-7-yl)-4-(substituted)phenylpyridine-3-carbonitrile (6a-m) were synthesized by multistep synthetic scheme (Figure 1). 2,3-Dihydroxynaphthalene on refluxing with phosphorus oxychloride yielded 2,3-dichloronaphthalene (1). Compound 1 on refluxing with ammonium chloride in excess of ammonia, catalytic amount of dil. HCl and ethanol undergoes amination (nucleophilic substitution) & furnished 2,3-diaminonaphthalene (2). Compound 2 on refluxing with equimolar quantity of benzil in ethanol undergoes nucleophilic addition followed by dehydration to yield 2,3-diphenylbenzo[g]quinoxaline (3). Compound 3 undergoes aromatic oxidation at C-5 and C-10 on refluxing with equimolar mixture of chromium trioxide and glacial acetic acid in water, till the permanent appearance of dark green colour, furnished 2,3-diphenylbenzo[g]quinoxaline-5,10-dione (4). Compound 4 on acetylation reaction with acetylchloride and anhydrous aluminium chloride in carbon tetrachloride yields 7-acetyl-2,3-diphenylbenzo[g]quinoxaline-5,10-dione (5). Compound 5 on refluxing with equimolar quantity of substituted aromatic aldehydes, ammonium acetate, malononitrile, ammonia and catalytic amount of dil. HCl undergoes Heitzch-Pyridine synthesis to yield 2-amino-6-(5,10-dioxo-2,3-diphenyl-5,10-dihydrobenzo[g]quinoxalin-7-yl)-4-(substituted) phenylnicotinonitrile (6a-m). Structure of all newly synthesized thirteen compounds were confirmed by FT-IR, 1H-NMR, MS-ESI spectral data interpretation and their elemental analysis.
Biological Activity (In-Vitro Antimycobacterial Screening)
The ten newly synthesized compounds viz. 6a, 6b, 6c, 6d, 6f, 6g, 6h, 6i, 6j and 6k were screened for their antimycobacterial screening against Mycobacterium tuberculosis H37Rv by L.J. Slope (Conventional) Method to observe the effect of substitution at phenyl ring attached to C-4 of pyridine ring of 2-amino-6-(5,10-dioxo-2,3-diphenyl-5,10-dihydrobenzo[g]quinoxalin-7-yl)-4-(substituted)phenylpyridine-3-carbonitrile (Compound 6a-m). Isoniazid and Rifampicin were used as standard drugs.
Unsubstituted phenyl ring (6a) on the 4-position of pyridine ring in above mentioned nucleus exhibited moderate antimycobacterial activity. Substitution with electron donating para-dimethylamino group (6h) on the phenyl ring present at C-4 of pyridine ring increases antimycobacterial activity and produces the most active antimycobacterial compound of the above mentioned newly synthesized series. Substitution with electron withdrawing 4-Cl group (6b) and 2-Cl (6g) at the phenyl ring also increases the antimycobacterial activity but lesser than that of substitution with para-dimethylamino group (6h). Substitution with para-hydroxy (6c) and electron withdrawing para-trifluoromethyl (6i) at the pheny ring exhibits moderate activity lesser than that of compounds 6h, 6g and 6b and more than that of compound with unsubstituted phenyl ring (6a). While, substitution with bulky electron withdrawing group para-nitro (6d), electron donating para-methyl (6k) and bulky electron donating groups 3-OH-4-OCH3 (6f) and 3,4-dimethoxy (6j) decreases the antimycobacterial activity to great extent and produces the least active compounds of the newly synthesized series.
From the above observations, Structure Activity Relationship (SAR) may be established as “substitution with smaller electron withdrawing and donating groups at the ortho and para positions of phenyl ring attached to C-4 of pyridine ring, present at 7-position of 2-amino-6-(5,10-dioxo-2,3-diphenyl-5,10-dihydrobenzo[g]quinoxalin-7-yl)-4-(substituted)phenylpyridine-3-carbonitrile increases antimycobacterial activity, while substitution with bulky electron donating groups at the phenyl ring attached to above mentioned position decreases antimycobacterial activity.”
Conclusion
Among the newly compounds synthesized compounds of series phenyl ring of 2-amino-6-(5,10-dioxo-2,3-diphenyl-5,10-dihydrobenzo[g]quinoxalin-7-yl)-4-(substituted)phenylpyridine-3-carbonitrile (6a-m), which were screened for antimycobacterial screening against Mycobacterium tuberculosis H37Rv by L. J. Slope (Conventional) Method, compound 6h with 4-N(CH3)2 at phenyl ring attached at pyridine group of 2-amino-6-(5,10-dioxo-2,3-diphenyl-5,10-dihydrobenzo[g]quinoxalin-7-yl)-4-(substituted)phenylpyridine-3-carbonitrile (6a-m) exhibited the maximal potency, while compounds with 4-Cl (6b) and 2-Cl (6g) substitutions at the above mentioned position exhibited better antimycobacterial potency as compared to other compounds. Compounds with 4-OH (6c), 4-CF3 (6i) and with no substitution (6a) at phenyl ring of the above mentioned position exhibited moderate antimycobacterial activity, while compounds with 3,4-(OCH3)2 (6j), 4-NO2 (6d), 3-OH-4-OCH3 (6f) and 4-CH3 (6k) showed minimal antimycobacterial potency against Mycobacterium tuberculosis H37Rv strain under observation.
Acknowledgement
Authors are grateful to Microcare Laboratory and TRC, Surat, Gujarat (India) for providing antimycobacterial activity data and Advanced Instrumentation Research Facilities (AIRF), Jawaharlal Nehru University, New Delhi, India for providing spectral data.
References
- Grande, F.; Aiello, F.; Grazia, O.D.; Brizzi, A.; Garofalo, A.; Neamati, N. Bioorg. Med. Chem., 2007, 15(1), 288-94.
CrossRef - Amin, K.M.; Ismail, M.M.F.; Noaman, E.; Soliman, D.H.; Ammar, Y.A. Bioorg. Med. Chem., 2006, 14, 6917-23.
CrossRef - Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Bioorg. Med. Chem., 2004, 12, 3711-21.
CrossRef
- Lee, H.; Cho, S.; Namgoong, K.; Jung, J.K.; Yang, S. Bioorg. Med. Chem. Lett., 2004, 14, 1235-37.
CrossRef - Diana, P.; Martorana, A.; Barraja, P.; Montalbaro, A. J. Med. Chem., 2008, 51 2387-99.
CrossRef - Chung, H.J.; Jung, O.J.; Chae, M.J.; Hong, S.Y.; Chung, K.H.; Lee, S.K. Bioorg. Med. Chem. Lett., 2005, 15, 3380-84.
CrossRef - Katsuyuki, A.; Obata, T.; Yamazaki, Y.; Mori, Y.; Hirokawa, H.; Koseki, J.; Hattori, T.; Niitsu, K.; Takeda, S.; Aburada, M.; Miyamoto, K. Chem. Pharm. Bull. 2007, 55, 255-67.
CrossRef - Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P.L.; Mollicotti, P.; Sechi, L.; Zanetti, S. Eur. J. Med. Chem., 2004, 39, 195-203.
CrossRef - Carta, A.; Paglietti, G.; Nikookar, M.E.R.; Sanna, P.; Sechi, L.; Zanetti, S. Eur. J. Med. Chem., 2002, 37, 355-66.
CrossRef - Tandon, V.K.; Yadav, D.B.; Maurya, H.K.; Chaturvedi, A.K.; Shukla, P.K. Bioorg. Med. Chem., 2006, 14, 6120-26.
CrossRef - Kotharkar, S.A.; Shinde, D.B. Bioorg. Med. Chem. Lett., 2006, 16, 6181-84.
CrossRef - Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Eur. J. Med. Chem., 2003, 38, 791-800.
CrossRef
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Bioorg. Med. Chem., 2003, 11, 2149-56.
CrossRef
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. J. Med. Chem., 2005, 48, 2019-25.
CrossRef - Seitz, L.E.; Suling, W.J.; Reynolds, R.C. J. Med. Chem., 2002, 45, 5604-06.
CrossRef
- WHO – Global Tuberculosis Report – 2015, 20th Edition, World Health Organization.
- Benoit; L.B.; Stewart, T.C. Antimycob. Agents Chemother., 2015, 59, 4457-63.
- Yano, T.; Kassovska, B.S.; The, J.S.; Winker, J.; Sullivan, K.; Issacs, A.; Schectiter, N.M.; Rubin. J. Biol. Chem., 2011, 286, 10276-87.
CrossRef - Winder, F.G.; Collins, P.B. J. Gen. Microbiol., 1970, 63, 41-48.
CrossRef - Quomard, A.; Lacove, C.; LanPelle, G. Antimicrob. Agents. Chemother., 1991, 35, 1035-39.
- Youatt, J.; Them, S.H. Am. Rev. Respir. Dis., 1969, 100, 25-30.
- Johnsson, K.; Schultz, P.G. J. Am. Chem. Soc., 1994, 116, 7425-26.
CrossRef - Johnsson K, King D S and Schultz P G 1995 J. Am. Chem. Soc. 117 5009-10.
CrossRef - Jensen, K.A. Bull. Int. Un. Tuberc., 1955, 25, 89-104.
- Canetti, G.; Froman, S.; Grosset, J.; Hauduroy, P.; Langerova. M.; Mahler, H.T.; Meissner, G.; Mitchison, D.A.; Sula, L. Bull. Wld. Hlth. Org., 1963, 29, 565-78.

This work is licensed under a Creative Commons Attribution 4.0 International License.









