Phytochemical Screening of Caralluma Lasiantha: Isolation of C21 Pregnane Steroid
Sireesha Malladi1, Venkata Nadh Ratnakaram2, Suresh Babu K3 and Pullaiah T4
1Department of Science and Humanities, Vignan’s University, Vadlamudi – 522213, India.
2GITAM University–Bengaluru Campus, Karnataka – 561203, India.
3Department of Chemistry, Mallareddy Engineering College, Hyderabad
4Department of Botany, SKD Universiy, Anantapur, India.
DOI : http://dx.doi.org/10.13005/ojc/330248
Article Received on : November 21, 2016
Article Accepted on : March 02, 2017
Phytochemical screening of caralluma lasiantha was carried out and one C21 pregnane steroid was isolated from chloroform extract. Based on spectroscopic studies (IR, 1H NMR, 13C NMR and ESI-MS) the isolated compound is 3β,14β-dihydroxy-14β-pregn-5-en-20-one which was earlier isolated from other species.
KEYWORDS:Caralluma lasiantha; Indian traditional medicine; C21 pregnane steroid; Chloroform extract; phytochemical screening
Download this article as:| Copy the following to cite this article: Malladi S, Ratnakaram V. N, Babu S. K, Pullaiah T. Phytochemical Screening of Caralluma Lasiantha: Isolation of C21 Pregnane Steroid. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Malladi S, Ratnakaram V. N, Babu S. K, Pullaiah T. Phytochemical Screening of Caralluma Lasiantha: Isolation of C21 Pregnane Steroid. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=30976 |
Introduction
In ancient system of medicine like ayurveda , unani and in folkaric medicine, several plants belongs to asclepiadaceae are proved to be helpful in healing of disorders. Asclepiadaceae family includes 200 genera and 2500 species. Caralluma is one of the genus of Asclepiadaceae which grows widely in dry places1. Certain species Caralluma were used as food in emergency needs in Pakistan and India2. The word ‘Caralluma’ is an Arabian word obtained from ‘qarh al-luhum’ means wound in the abscess or flesh3. Caralluma is also spoken as the synonym of Boucerosia, but it varies from Boucerosia by its floral arrangement3. In India species of Caralluma are found to be palatable and also considered as a component of traditional medical system. Now a days Caralluma is gaining much significance from scientists as it exhibits immunostimulating activities because of presence of flavanoids and saponins4. Caralluma umbellata Haw also used to treat stomach disorder and pain, which can be evidenced by good antibacterial activity of Caralluma umbellata extracts5.
Caralluma lasiantha (syn. Boucerosia lasiantha) is well known different local names like Kundeti Kommulu in Telugu and Sirumankeerai in Tamil6. It is a member of Asclepiadaceae family. It is succulent in habit and a familiar indoor ornamental plant7. It growth is found in surrounding places of Anantapur and Chittoor, Andhra Pradesh, India. To reduce body heat, it is used in Indian traditional medicine8. Differentiation among various species of Caralluma genus is more difficult because of their more intermediary forms in their habitation9. Thus, Pavan Kumar et al10 suggested standardized parameters for right differentiation of four Caralluma species to determine genuinely. A review article on C.lasiantha was published by us11. Anti-proliferative property of Boucerosia lasiantha (methanolic extracts) against A431 human skin cancer cells and A375 human malignant melanoma was reported by Madhuri et al12. Madhuri and Siva Rama Krishna13 reported the antioxidant activity of methanolic extracts of Boucerosia lasiantha. Antihyperglycemic / hypoglycemic activitt of methanolic extracts of Caralluma lasiantha were investigated by Harsha Kumar14. In our latest studies, antibacterial activity of extracts of C. lasiantha was reported against both Gram (-) bacteria and Gram (+) bacteria 15. Likewise, depending on antifungal activity of extracts of Caralluma lasiantha against tested fungi, C. lasiantha was recommended as alternate to manage storage fungi by us16. Ramesh et al17 reported two bisdesmosidic C-21 steroidal glycosides (lasianthoside-A and lasianthoside-B) from C. lasiantha (Fig.1) and a known flavone glycoside (Luteoline-4-O-neohesperiodoside) (Fig.2). Steroidal glycosides were extracted from C.lasiantha in their studies, using polar solvents like alcohols18. We have reported first time the isolation of stigmasterol from C.lasiantha19. Thorough literature shows that no extractions from C.lasiantha were carried out using solvents having intermediate polarity. Hence, phytochemical screening of chloroform extracts of C.lasiantha is carried out in the present study.
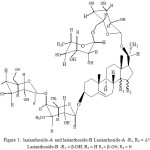 |
Figure 1: lasianthoside-A and lasianthoside-B Lasianthoside-A -R1, R2 = D14-15, Lasianthoside-B -R1 = β-OH,R2 = H R1 = β-OH, R2 = H |
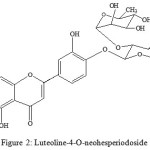 |
Figure 2: Luteoline-4-O-neohesperiodoside |
Materials and Methods
All chemicals used in the present studies are Analytical Reagent grade, Merck India Co. Ltd. If necessary, chemicals are purified by using standard procedures. Geographical location20, plant collection21 and season22 affect the active constituents of the plants which play an important role in exhibiting the biological activities by extracts of plants. Fresh plants of Caralluma lasiantha (Asclepiadaceae) are collected from Gooty, Anantapur District, Andhra Pradesh, India in February 2012. A voucher specimen of it was deposited in Herbarium, Department of Botany, Sri Krishna Devaraya University, Anantapur.
Isolation and identification
Stems and roots were dried under shade, powdered and sieved (sieve No.14). Then the powder was stored in air tight containers. The weighed quantity of powder was extracted with successive solvent extraction in soxhlet extractor by using solvents of varying polarity (Hexane, Chloroform, and Methanol). Last traces of solvent were removed by applying vacuum to concentrate all the extracts 23, 24. Later, the crude extracts are purified by recrystallization. Melting point was recorded on a Fisher–John apparatus. The IR spectra were recorded on an IFS-120H spectrometer. The 1H NMR and 13C NMR spectra were obtained on Bruker 300MHz, 75MHz spectrometer, using TMS as an internal standard. ESI-MS was recorded on a ZAB-HS mass spectrometer and HREIMS was recorded on the Agilent Technologies 6510 Q-TOF LC/MS.
Spectral Data
I.R.: vmax 3489, 2925, 2854, 1678 and 1458 cm-1
1H NMR (CDCl3): δ 5.42 (1H, t), 4.46 (1H, s), 3.53 (1H, s), 2.94 (1H, t), 2.259 (3H, s), 1.84 (1H, t), complex pattern peaks at δ 1.50, 1.27 and 1.0.
13C NMR (CDCl3): δ 206.4 (C-20), 138.9 (C-5), 121.3 (C-6), 84.3 (C-14), 71.4 (C-3), 62.8 (C-17), 48.6 (C-13), 45.5 (C-9), 40.7 (C-4), 38.5 (C-12), 36.4 (C-8), 35.9 (C-15), 33.9 (C-1), 32.7 (C-21), 31.2 (C-2), 37.0 (C-10), 26.8 (C-7), 24.0 (C-16), 20.4 (C-11), 19.6 (C-19), 15.0 (C-18)
Mass (ESI-MS )
(m/z) 355 (M+Na)+, 315.2, 301, 297.2, 279, 199, 139, 101.1
Libermann-Burchard Test
The extract was boiled after treating with few drops of acetic anhydride. Addition of concentrated sulphuric acid to the above cooled solution, a brown ring was formed at the junction of two layers as well as the upper layer forms green colour to confirm the presence of sterols25.
Results and Discussion
Nature of extractable phytochemical depends on the polarity of solvents 26. In the present study, chloroform extracts were taken. A positive test of Libermann-Burchard test exhibits the presence of sterols in the crude mixture25. By repetitive column chromatography, the crude extract of C. lasiantha (using petroleum ether as a solvent) was purified using silica gel (230-400 mesh) and a mixture of petroleum ether and acetone as an eluent. An amorphous white solid compound (M.Pt: 190-200°C) was obtained as one of the product from the mixture. The isolated phytochemical was confirmed as steroid from a positive TLC test. The spot on TLC plate was eluted with a solvent mixture of hexane; ethyl acetate (7:35) and sprayed with 10% sulphuric acid to give a dark pink spot. Rf value was found to be 0.42.
I.R. spectrum shows the presence of carbonyl group (C=O str: 1678 cm-1), skeletal unsaturation (C=C str: 1458 cm-1) and a hydroxyl group (O-H str: 3489 cm-1). In the 1H-NMR spectrum, one singlet is observed at δ 1.0 due to superimposed signal of methyl protons present on C18 and C19. A triplet at δ 5.4 explains the presence of hydrogen on unsaturated carbon (C6). Two singlets at higher δ values (4.46 and δ 3.53) show the attachment of an electronegative atom like oxygen to proton indicating the presence of a hydroxyl group attached to C14 and C3 respectively. CH proton (i.e., H attached to C3 which is linked with hydroxyl group) absorbed at δ 2.94. CH3 and CH protons attached to carbonyl group shown peaks at δ 2.26 and d 1.84 respectively.
Spectral data of 13C-NMR spectrum supported the proposed structure (Fig.3). A peak above δ 200 (δ 206.4) shows the presence of a carbonyl group. Peaks in between δ 120 to 140 (138.9 and 121.3) exhibits the presence of unsaturation between C5 and C6. Presence of peaks in the higher side of alkanes absorption range (δ 0 to 80) at 84.3 and 71.4 shows the attachment of hydroxyl group to C14 and C17 respectively. From elemental analysis (C-75%; H-9.6%; O-14.4%) and mass analysis (m/z: M+Na+ = 355), molecular formula was found to be C21H32O3. The isolated compound was recognized as a typical C21 steroidal skeleton with a carbonyl group. On ESI-MS fragmentation, a series of product ions were generated at m/z 315.2, 301, 297.2, 279, 199, 139, 101.1 which explain the fragmentation pattern including loss of hydroxyl group in the form of water molecules.
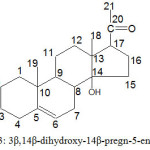 |
Figure 3: 3β,14β-dihydroxy-14β-pregn-5-en-20-one |
The structure of isolated compound is proposed as 3β,14β-dihydroxy-14β-pregn-5-en-20-one (Fig.3) by comparing its spectral data with those available in literature 27, 28, 29. The present C21 steroid was isolated first from Cynanchum paniculatam 27. Based on its presence in non-polar extracts of Caralluma umbellata, Ramesh et al29 suggested that it is the precursor for glycosides like carumbelloside I & II. It was also concluded that the present C21 steroid can be helpful as a marker for Caralluma genus. As different moieties and functional groups present on synthetic / natural molecules direct their pharmacological activities 30-35, structure activity relationship between such activities and this isolated molecule can be further studied.
Conclusion
C21 steroid was isolated from chloroform extract of Caralluma lasiantha. The isolated compound is 3β,14β-dihydroxy-14β-pregn-5-en-20-one which was earlier isolated from Cynanchum paniculatam and Caralluma umbellata.
References
- Rajendra. ; Ramaswam. ; Kamala. USP filed – 4. 2004, 6376657.
- Atal, C.K. ; Sharma, B.M. ; Bhatia, A.K. The Indian Forester. 1980, 106, 211-219.
- Adnan, M. ; Jan, S. ; Mussarat, S. ; Tariq, A. ; Begum, S. ; Afroz, A. ; Shinwari, Z.K. J. Pharm. Pharmacol . 2014, 66, 1351-1368.
CrossRef - Kamil, M.A. ; Fjayaraj, F. ; Ahmad, C. ; Gunasekhar, S. J. Pharm. Pharmacol. 1999, 51, 225-229.
- Babu, K.S. ; Malladi, S. ; Nadh, R.V. ; Rambabu, S.S. Annu. Res. Rev. Biol. 2014, 4, 840-855.
CrossRef - Arinathan, V. ; Mohan, V.R. ; Debritto, A.J. ; Murugan, C. Indian. J. Traditional. Knowledge. 2007, 6, 163-168.
- Reddy, S.R. ; Reddy, A.M. ; Yasodamma, N. Indian. J. Fundam. Appl. Life. Sci, 2012, 2, 192-199.
- Vikneshwaran, D. ; Viji, M. ; Rajalakshmi, K. Ethnobotanical. Leaflets, 12, 1108-1115.
- Madhuri, V. ; Amrutha, V.A. ; Murthy, K.S.R. Asian. J. Phar. Biol. Res. 2011, 1, 500-507.
- Pavan K.B. ; Ashok, G. ; Ibrahim, M. ; Ramachandra N.M. ; Rashmi K.P. J. Phytopharmacol. 2015, 4, 34-40.
- M. Sireesha, K. Suresh babu, R. Venkata Nadh and T. Pullaiah, Caralluma lasiantha: A review on it’s vital role in Indian traditional medicine, Accepted for Res.J.Pharm.Bio.Chem.Sc, 2017.,8(1).
- Madhuri, V. ; Murthy, K.S.R. ; Amrutha, V.A. ; Siva Rama Krishna, C. Eur. J. Exp. Biol 2014. 4, 160-167.
- Madhuri, V. ; Siva Rama Krishna, C. Int. J. Appl. Sci. Biotechnol. 2014, 2, 83-87.
- Harsha, K.V. Int. J. Pharm. Sci. Res. 2016, 7, 2525-2530.
- Sireesha, M. ; Suresh babu, K. ; Venkata Nadh, R. ; Pullaiah, T. Antibacterial Activity of Caralluma lasiantha (communicated)
- Sireesha, M. ; Suresh babu, K. ; Venkata Nadh, R. ; Pullaiah, T. Antifungal Activity of Caralluma lasiantha (communicated).
- Ramesh, M. ; Rao, Y.N. ; Kumar, M.R. ; Rao, A.V.N.A. ; Prabhakar, M.C. ; Reddy, B.M. J. Ethnopharmacol. 1999, 68, 349-352.
CrossRef - Qiu, S.X. ; Cordell, G.A. ; Kumar, B.R. ; Rao, Y.N. ; Ramesh, M. ; Kokate, C. ; Rao, A.V.N.A. Phytochem. 1999, 50, 485-491.
CrossRef - M. Sireesha, K. Suresh babu, R. Venkata Nadh and T. Pullaiah, Phytochemical investigation of Caralluma lasiantha: Isolation of stigmasterol, an active immunomodulatory agent (Communicated).
- Adoum, O.A. ; Akinniyi, J.A. ; Omar, T. R. Br. Ann. Borno. 1997, 13, 199-207.
- Odugbemi, T. ed. Outlines and Pictures of Medinal Plants from Nigeria. Tolu Odugbemi, 2008.
- World Health Organization. 2003. Traditional medicine. Fact sheet Number 134.
- Trease, G.E. ; Evans, W.C. Pharmacognosy, Saunders. Elsevier, Amsterdam, The Netherlands. 2002, 36-51.
- Gupta, A.K. Introduction to Pharmaceutics-1. CBS publication, New Delhi. 2004.
- 25. Edeoga, H. O. ; Okwu, D. E. ; Mbaebie, B. O. Afr. J. Biotechnol. 2005, 4, 685-688.
CrossRef - Marjorie, M.C. Clin. Microbial. Rev. 1999, 12, 564-582.
CrossRef - Sugama, K. ; Hayashi, K. ; Mitsuhashi, H. ; Kaneko, K. Chem. Pharm. Bull. 1986. 34, 4500-4507.
CrossRef - Lin, L.J. ; Lin L.Z. ; Roberto, R.G. ; Geoffrey, A. ; Cordell, G.A. ; Ramesh, M. ; Srilatha, B. ; Reddy, B.; Rao, A.V.N.A. Phytochem. 1994, 35, 1549-1553.
CrossRef - Ramesh, M. ; Ravi, K.B. ; Kokate, C. ; Rao, A.V.N.A. Nat. Prod. Sci. 2005, 11, 115-117.
- Sudhir, M.S. ; Nadh, R.V. Bulg. Chem. Commun. 2014, 46, 25 – 30.
- Sudhir, M.S. ; Nadh, R.V. Radhika, S. Drug Invention Today. 2013, 5, 126-132.
CrossRef - Sudhir, M.S. ; Nadh, R.V. J. Pharm.Res. 2013; 7: 47-52.
CrossRef - Suresh, G. ; Nadh, R.V. ; Srinivasu, N. ; Kaushal, K. Synth. Commun. 2016 doi: 10.1080/00397911.2016.1242748.
CrossRef - Khalil, O.M. ; Refaat, H.M. Orient. J. Chem. 2011, 27,1581-1590.
- Azab, I.H.E. ; Break, L.M. ; El-Zahrani, Z.A. A. Orient. J. Chem. 2016. 32, 2435-2449.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









