Calculation of Spin Orbit Coupling of Tungsten (III) Complexes: A DFT Application
M. L. Sehgal
Fmr. Head, Department of Chemistry, D.A.V. College, Jalandhar-144008, India.
Corresponding Author E-mail: mehjabeenjaved200@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330263
Making use of DFT, we could calculate Spin Orbit Coupling Constant (lcomplex) values of the five tungsten (III) complexes which were difficult to arrive at experimentally since there would always occur errors during the determination of their contributing parameters like CFSE and (Aten).
KEYWORDS:Coupling; Stabilization;
Download this article as:| Copy the following to cite this article: Sehgal M. L. Calculation of Spin Orbit Coupling of Tungsten (III) Complexes: A DFT Applicationz. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Sehgal M. L. Calculation of Spin Orbit Coupling of Tungsten (III) Complexes: A DFT Applicationz. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31488 |
Introduction
Unlike the 1st transition series metal ion complexes, there had, hardly, been any study done on the calculation of Spin Orbit Coupling (λcomplex) of the corresponding complexes of 2nd and 3rd transition series. The limitation would arise because high λcomplex values( 1 ) of their complexes caused errors both in the exact determination of their Crystal Field Stabilization Energies (CFSE) as well as the ESR parameters (especially A ten).They would, further, cause errors in the g values if determined by experimental methods. We applied Density Functional Theory (DFT) implemented in ADF 2010.02 software to its ESR/EPR Program which was run by giving Single Point, LDA, Default, Spin Orbit, Unrestricted, None, Collinear and ZORA commands using TZP Basis set with Nosym symmetry after definite Pre-optimization of five W (III) complexes to obtain their g iso values (2-4). Five known relations (a-e) were used in a sequence. Magnetic moments (mADF) arising from the First Order Zeeman Effect were calculated from the g iso values (a) . Magnetic moment values arising from the Second Order Zeeman Effect [Temperature Independent Paramagnetic Moments (m tip)] were calculated by (c) from their paramagnetic susceptibilities (χtip) as calculated by (b). Sum of mADF and m tip would give effective magnetic moments (μeff) (d) .CFSE values of W (III) complexes were ≈1.75 times the reported CFSE values of the corresponding Cr (III) complexes (5-7).λComplex values (8) of W (III) complexes were calculated by (e).
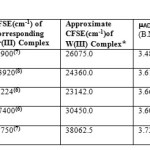 |
Table 1 Click here to View table |
*Multiply CFSE of corresponding Cr (III) Complex by 1.75
**Multiply by 106- c g s
μs.o=3.8729 B.M and χs. o. =6250 * 10– 6cgs for 3 unpaired electrons
(a)μADF = [giso2s(s+1)]1/2
(b) χtip = 8Nb2/10Dq
(c) μtip= χtip * ms.o /cs.o
(d) μt = mADF+m tip
(e) μeff =mso (1- σ * λ complex /10Dq)
References
- Figgis, B. N. Intro. Ligand. Fields. ed. U.S; Table 3.4, 1966, 60.
- Singh, H.; Bhardwaj, A. K.; Sehgal, M. L.; Mittal, S. K. Int. J. Current Res. Rev. 2012, 4, 12-28
- Singh, H.; Bhardwaj, A. K.; Sehgal, M. L.; Mittal, S. K. Int. J. Current Res. Rev. 2013, 5, 13-31
- Singh, H.; Bhardwaj, A. K.; Sehgal, M. L.; Mittal, S. K. Int. J. Current Res. Rev 2013, 5, 71- 88.
- Jorgensen, C. K. Absorp. Spectra. Chem. Bond. Compl. Paragon Press, N .Y. 1962.
- Jorgensen, C. K. Advn. Chem. Phys. 1963, 5, 33.
- Hatfield, W.E.; Fay, R.C.; Pfluger, C.E.; Piper, T. S. J. Am .Chem.Soc. 1963, 85, 265.
CrossRef - Alan, E. Intro. Magneto. Chem. 1968.

This work is licensed under a Creative Commons Attribution 4.0 International License.









