Synthesis of Some Pyrazole containing Chalcones and Pyridine-3-Carbonitirles and Study of their Anti-inflammatory Activity
Anil G. Gadhave and Bhagwat K. Uphade
Department of Chemistry and Research Centre, Padmashri Vikhe Patil College, Pravaranagar, Dist-Ahmednagar-413713, India.
Corresponding Author E-mail: anilgadhave@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330125
In present work the 4-formyl pyrazole (1) were treated with 4-morpholinoacetophene (2) in the presence of aqueous KOH to give Chalcones (3). The chalcones are subjected to react with malononitrile using sodium methoxide as a base in methanol to give pyridine-3-carbonitriles (4). The synthesized chalcone and pyridine-3-carbonitrile derivatives were subjected for study of their anti-inflammatory activity by the well known carrageenan induced rat paw edema method. The products 3a, 3e and 4e exhibited good activity as compared with standard diclofenac drug. The formation of all compounds was established by the spectral techniques such as Infra Red, 1H-NMR and mass.
KEYWORDS:anti-inflammatory; pyrazole; pridine-3-carbonitirle; chalcone
Download this article as:| Copy the following to cite this article: Gadhave A. G, Uphade B. K. Synthesis of Some Pyrazole containing Chalcones and Pyridine-3-Carbonitirles and Study of their Anti-inflammatory Activity. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Gadhave A. G, Uphade B. K. Synthesis of Some Pyrazole containing Chalcones and Pyridine-3-Carbonitirles and Study of their Anti-inflammatory Activity. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30450 |
Introduction
In the last few decades research has progressed in understanding the mechanism of inflammation which is a physiological process essential for the survival but it can also cause human morbidity and mortality1,2. The disorders related to pain cannot be easily identified or removed. The current available drugs which possess anti-inflammatory activity are known to have some boundaries in medical use that are ulceration and gastrointestinal haemorrhage, addiction and acceptance for opiates3. These limitations creates some challenges for organic and medicinal chemist to design and develop more useful anti-inflammatory drugs4,5. The efforts for improving the pharmacological profile of anti-inflammatory agents led towards discovery of multiple isoform of cyclooxygenase (COX)6.
Chalcones are the biologically active scaffold used in synthesis of various heterocyclic compounds. They are also known to possess pharmacological activites such as tyrosine inhibition7, anti-inflammatory8, anticancer9, antioxidant10 and nitric oxide inhibition11. The incorporation of pyrazole moiety into heterocyclic compounds gave the pharmaceutical drugs such as celecoxib12 and rimonabant13. They also possess biological activites such as anti-inflammatory14, A3 adenosine receptor antagonist15, antiviral16 and antifungal17. The N-functionalized morpholine containing heterocyclic compounds are biologically important motifs which are known to possess antidiabetic18, antiemetic19, anti-inflammatory and central nervous system20 activities.
The pyridine is a heterocyclic compound found in variety of natural and synthetic products possessing pharmacological activities such as hypoglycemic, dopamine transporter inhibitors and anti-inflammatory21,22. They also have application in dyes for synthetic fabrics23, light emitting24 and electroluminescence25 devices.
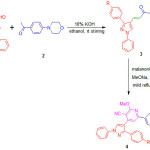 |
Scheme 1 |
The biological applications of chalcone, pyridine, pyrazole, morpholine and in continuation to our ongoing research26-29 herein we report the synthesis of chalcones, pyridine-3-carbonitriles and their anti-inflammatory activity.
Materials and Methods
The chemicals used were of analytical grade and are obtained commercially. The M. P. of all products was performed in open capillaries. The Shimadzu FTIR spectrophotometer was used for the Infra Red Spectrum (IR) using KBr and only functional group frequencies are given in cm-1. The Proton Magnetic Resonance (PMR) spectrum was carried over Bruker Avance spectrometer (400 MHz) using TMS as an internal standard and CDCl3 solvent. The Finnigan mass spectrometer was used for Mass spectrum. The TLC plates available commercially were used to check the progress and completion of reactions.
General protocol for synthesis of 1,3-disubstituted prop-2-en-1-one (chalcones): (3a-e)
The 4-formyl pyrazole (10 mmol) and 4-morpholinoacetophenone (10 mmol) were taken in R. B. flask and to it 20 ml of EtOH was added. To the same flask 10 ml of 20% KOH solution was added. The resulting reaction content was allowed to attain room temperature and stirred for 24 h. The progress and completion of reaction was monitored by TLC and then the reaction contents were added in ice and neutralized by acetic acid. The precipitated product was separated by filtration and dried. The product was purified by recrystallization using ethanol to furnish chalcones (3a-e). The characterization data of synthesized compounds is as follows.
Compound 3a
Yield 70%, m.p. 282-283oC; IR (KBr, cm-1): 1688 (Carbonyl), 1654 (C=N), 1625 (C=C), 1235 (C-O); 1H NMR (CDCl3, δ): 3.35 (triplet, 4H, -N-CH2-), 3.70 (triplet, 4H, -O-CH2-), 6.94 (doublet, 2H, J=8 Hz, H-Aromatic), 7.31-7.87 (multiplet, 12H, H-Aromatic & CH=CH), 7.95 (d, 2H, J=8 Hz, H-Aromatic), 8.22 (s, 1H, proton of pyrazole ring); Mass m/z 435 [M]+.
Compound 3b
Yield 77%, m.p. 252-253oC; IR (KBr, cm-1): 1690 (Carbonyl), 1648 (C=N), 1627 (C=C), 1230 (C-O); 1H NMR (CDCl3, δ): 2.17 (singlet, 3H, CH3 of Aromatic ring), 3.45 (triplet, 4H, -N-CH2-), 3.90 (triplet, 4H, -O-CH2-), 7.08 (doublet, 2H, J=8 Hz, H-Aromatic), 7.28-7.98 (multiplet, 11H, H-Aromatic & CH=CH), 8.01 (doublet, 2H, J=8 Hz, H-Aromatic), 8.35 (singlet, 1H, proton of pyrazole ring); Mass m/z 449 [M]+.
Compound 3c
Yield 73%, m.p. 220-221oC; IR (KBr, cm-1): 1685 (Carbonyl), 1652 (C=N), 1620 (C=C), 1222 (C-O), 1090 (Ar-Cl); 1H NMR (CDCl3, δ): 3.32 (triplet, 4H, -N-CH2-), 3.83 (triplet, 4H, -O-CH2-), 6.91 (doublet, 2H, J=8 Hz, H-Aromatic), 7.34-7.84 (multiplet, 11H, H-Aromatic & CH=CH), 7.94 (doublet, 2H, J=8 Hz, H-Aromatic), 8.33 (singlet, 1H, proton of pyrazole ring); Mass m/z 469 [M]+.
Compound 3d
Yield 69%, m.p. 230-231oC; IR (KBr, cm-1): 1687 (Carbonyl), 1649 (C=N), 1632 (C=C), 1244 (C-O), 1188 (Ar-F); 1H NMR (CDCl3, δ): 3.32 (triplet, 4H, -N-CH2-), 3.87 (triplet, 4H, -O-CH2-), 6.89 (doublet, 2H, J=8 Hz, H-Aromatic), 7.16-7.88 (multiplet, 11H, H-Aromatic & CH=CH), 7.93 (doublet, 2H, J=8 Hz, H-Aromatic), 8.33 (singlet, 1H, proton of pyrazole ring); Mass m/z 453 [M]+.
Compound 3e
Yield 60%, m.p. 238-240oC; IR (KBr, cm-1): 1680 (Carbonyl), 1653 (C=N), 1619 (C=C), 1216 (C-O); 1H NMR (CDCl3, δ): 3.31 (triplet, 4H, -N-CH2-), 3.84 (multiplet, 4H of -O-CH2– & 3H of Aromatic-methoxy group proton), 6.90 (doublet, 2H, J=8 Hz, H-Aromatic), 7.00-7.89 (multiplet, 11H, H-Aromatic & CH=CH), 7.95 (doublet, 2H, J=8 Hz, H-Aromatic), 8.32 (singlet, 1H, proton of pyrazole ring); Mass m/z 465 [M]+.
General protocol for synthesis of pyridine-3-carbonitriles (4a-e)
The chalcone 3 (1 mmol) and malononitrile (1 mmol) was taken in methanol (10 mL) in a R. B. flask. To this sodium methoxide was added (prepared freshly from sodium metal and absolute methanol) and the reaction contents were refluxed for 3-4 h. The progress and completion of reaction was checked by TLC. Then the reaction contents were allowed to attain the room temperature. The precipitated product was filtered, washed with water and dried. The products were purified by recrystallization using ethanol to furnish pure pyridine-3-carbonitrile derivatives (4a-e). The characterization data of synthesized compounds is as follows.
Compound 4a
Yield 81%, m.p. 259-260oC; IR (KBr, cm-1): 2230 (CN), 1660 (C=N), 1601 (C=C), 1235 (C-O); 1H NMR (CDCl3, δ): 3.32 (triplet, 4H, -N-CH2-), 3.86 (triplet, 4H of -O-CH2-), 4.17 (singlet, 3H, Aromatic-methoxy group proton), 6.89 (doublet, 2H, J=8 Hz, H-Aromatic), 7.34-7.90 (multiplet, 11H, H-Aromatic), 7.93 (doublet, 2H, J=8 Hz, H-Aromatic), 8.52 (singlet, 1H, proton of pyrazole ring); Mass m/z 513 [M]+.
Compound 4b
Yield 78%, m.p. 286-287oC; IR (KBr, cm-1): 2235 (CN), 1647 (C=N), 1595 (C=C), 1241 (C-O); 1H NMR (CDCl3, δ): 2.39 (singlet, 3H, CH3 of Aromatic ring), 3.25 (triplet, 4H, -N-CH2-), 3.88 (triplet, 4H of -O-CH2-), 4.17 (singlet, 3H, Aromatic-methoxy group proton), 6.92 (doublet, 2H, J=8 Hz, H-Aromatic), 7.12 (singlet, 1H, H-Aromatic), 7.19-7.73 (multiplet, 9H, H-Aromatic), 7.79 (doublet, 2H, J=8 Hz, H-Aromatic), 8.48 (singlet, 1H, proton of pyrazole ring); Mass m/z 527 [M]+.
Compound 4c
Yield 82%, m.p. 262-263oC; IR (KBr, cm-1): 2216 (CN), 1657 (C=N), 1599 (C=C), 1255 (C-O), 1075 (Ar-Cl); 1H NMR (CDCl3, δ): 3.29 (triplet, 4H, -N-CH2-), 3.92 (triplet, 4H of -O-CH2-), 4.18 (singlet, 3H, Aromatic-methoxy group proton), 7.02 (doublet, 2H, J=8 Hz, H-Aromatic), 7.11 (singlet, 1H, H-Aromatic), 7.35-7.81 (multiplet, 11H, H-Aromatic), 8.46 (singlet, 1H, proton of pyrazole ring); Mass m/z 548 [M]+.
Compound 4d
Yield 75%, m.p. 258-259oC; IR (KBr, cm-1): 2221 (CN), 1650 (C=N), 1605 (C=C), 1232 (C-O), 1126 (Ar-F); 1H NMR (CDCl3, δ): 3.25 (triplet, 4H, -N-CH2-), 3.86 (triplet, 4H of -O-CH2-), 4.18 (singlet, 3H, Aromatic-methoxy group proton), 6.90 (doublet, 2H, J=8 Hz, H-Aromatic), 7.07 (singlet, 1H, H-Aromatic), 7.09-7.81 (multiplet, 11H, H-Aromatic), 8.47 (singlet, 1H, proton of pyrazole ring); Mass m/z 531 [M]+.
Compound 4e
Yield 71%, m.p. 260-261oC; IR (KBr, cm-1): 2215 (CN), 1662 (C=N), 1610 (C=C), 1254 (C-O); 1H NMR (CDCl3, δ): 3.24 (triplet, 4H, -N-CH2-), 3.82 (triplet, 4H of -O-CH2-), 3.95 (singlet, 3H, Aromatic-methoxy group proton), 4.19 (singlet, 3H, Aromatic-methoxy group proton), 6.99 (doublet, 2H, J=8 Hz, H-Aromatic), 7.10 (singlet, 1H, H-Aromatic), 7.31-7.82 (multiplet, 11H, H-Aromatic), 8.49 (singlet, 1H, proton of pyrazole ring); Mass m/z 543 [M]+.
Anti-inflammatory activity study
The chalcones and pyridine-3-carbonitriles were subjected for anti-inflammatory activity study by literature known protocol using carrageenan-induced paw edema test in rats. The study was carried using wistar rats of 150-200 g weight. Standard pellet diet and water ad libitum was fed to the study animals. The 12 h light and 12 h dark cycle at 25oC was maintained for the animals. Animal experiment was approved by IAEC/ CPCSEA. The overnight fasted rats were grouped in three animals per group. The control group was given vehicle (1% CMC in water in a volume of 10 mL/kg) only. In vehicle (10 mL/kg) the Diclofenac (10 mg/kg) and test samples (10 mg/kg) was suspended and administered orally to the respective groups. After half hour, 0.1 ml of 1% carrageenan solution (prepared in normal saline) was injected in the subplanter region of right hind paw of all animals. After 3 hours, with the help of digital vernier caliper (Mitutoyo, Japan) the edema formed in the paw was measured. Edema induced by carrageenan alone was considered as 100% induction. Any major decrease in paw volume compared to control group was measured as anti-inflammatory response. The outcome obtained during the study is presented in Table 2
Results and Discussion
The synthesis of chalcones and pyridine-3-carbonitrile derivatives are shown in scheme 1. The chalcones 3a-e is synthesized by well known Claisen-Schmidt condensation of 4-formyl pyrazole 1a-e with 4-morpholinoacetophenone 2 in presence KOH base and ethanol as a solvent under room temperature stirring. The formation of chalcones 3a-e was confirmed on the basis of the spectral techniques. The Infra Red spectrum of chalcone 3a show characteristic stretching frequencies at 1688 cm-1 for C=O, 1654 cm-1 for C=N, 1625 cm-1 for C=C and 1235 cm-1 for C-O groups. The 1H-NMR spectra of compound 3c shows two triplets at 3.32 δ and 3.83 δ each for four protons are due to -N-CH2– and -O-CH2– groups of morpholine ring respectively. The aromatic as well as C=C group protons appeared in 6.91-7.94 δ while the pyrazole ring proton exhibited singlet at 8.33 δ. The mass spectrum of compound 3e shows m/z 465 peak corresponding to its molecular weight.
Table 1: The physical data of synthesized chalcones and pyridine-3-carbonitriles
|
Compound |
R Group |
Molecular Formula |
Molecular Weight (gm) |
Melting Point (oC) |
Yield (%) |
|
3a |
H |
C28H25N3O2 |
435.51 |
282-283 |
70 |
|
3b |
4-Me |
C29H27N3O2 |
449.54 |
252-253 |
77 |
|
3c |
4-Cl |
C28H24ClN3O2 |
469.96 |
220-221 |
73 |
|
3d |
4-F |
C28H24FN3O2 |
453.50 |
230-231 |
69 |
|
3e |
4-OMe |
C29H27N3O3 |
465.54 |
238-239 |
60 |
|
4a |
H |
C32H27N5O2 |
513.58 |
259-260 |
81 |
|
4b |
4-Me |
C33H29N5O2 |
527.61 |
286-287 |
78 |
|
4c |
4-Cl |
C32H26ClN5O2 |
548.03 |
262-263 |
82 |
|
4d |
4-F |
C32H26FN5O2 |
531.57 |
258-259 |
75 |
|
4e |
4-OMe |
C33H29N5O3 |
543.61 |
260-261 |
71 |
Table 2: The anti-inflammatory activity study results of chalcones and pyridine-3-carbonitriles
|
Compound |
R |
Control (paw volume in mm) |
Test (paw volume in mm) |
Difference |
|
Control |
— |
9.8 |
— |
— |
|
Diclofenac |
— |
9.8 |
8.1 |
1.7 |
|
3a |
H |
9.8 |
8.2 |
1.6 |
|
3b |
4-Me |
9.8 |
9.1 |
0.7 |
|
3c |
4-Cl |
9.8 |
9.2 |
0.6 |
|
3d |
4-F |
9.8 |
9.1 |
0.7 |
|
3e |
4-OMe |
9.8 |
7.8 |
2.0 |
|
4a |
H |
9.8 |
9.3 |
0.5 |
|
4b |
4-Me |
9.8 |
9.2 |
0.6 |
|
4c |
4-Cl |
9.8 |
9.2 |
0.6 |
|
4d |
4-F |
9.8 |
9.1 |
0.7 |
|
4e |
4-OMe |
9.8 |
8.5 |
1.3 |
The chalcones react with malononitrile in methanol solvent in the presence of freshly prepared sodium methoxide base under reflux condition gave pyridine-3-carbonotriles derivatives 4a-e. The formation of pyridine-3-carbonotriles was confirmed on the basis of spectral techniques. The Infra Red spectra of compound 4b exhibited peaks at 2235 cm-1 for CN and 1647 cm-1 due to C=N functional groups. The 1H-NMR spectrum of compound 4d exhibited two triplets at 3.25 δ and 3.86 δ each for four protons are due to -N-CH2– and -O-CH2– groups of morpholine ring respectively. The new signal appeared at 4.18 δ as a singlet for three protons is due to -OCH3 group. The aromatic ring protons show multiplet between 6.90-7.81 δ. The proton of pyrazole ring shows a singlet at 8.47 δ. The mass spectrum of compound 4e showed m/z 543 peak corresponding to its molecular weight which confirm its formation.
The anti-inflammatory activity study results are represented in Table no. 2 which reveals that, the compound 3a, 3e and 4e exhibited good activity as compared with standard diclofenac drug. The other compounds possess moderate activity profile. The good biological activity in chalcone 3a and 3e are attributed due to the no substituent in 3a and para methoxy substituent in 3e on phenyl ring of pyrazole moiety. The 4e pyridine-3-carbonitrile possesses methoxy substituent on phenyl ring of pyrazole which may have enhanced its activity.
Owing to the biological importance associated with pyrazole, morpholine, chalcone and pyridine-3-carbonitriles scaffolds, herein we reports the synthesis, characterization and anti-inflammatory activity study of morpholine and pyrazole containing chalcones and pyridine-3-carbonitriles derivatives. The insight obtained in the present study will be useful for development of newer biologically important compounds.
Acknowledgements
The authors are grateful to Pravara Rural Education Society, Pravaranagar (MS) and Principal of our college for providing all necessary facilities. We are also thankful to Prin. Dr. B. K. Karale for the encouragement and valuable guidance. We are also thankful to SAIF, Panjab University, Chandigarh for spectral analysis. Authors are thankful to BCUD, Savitribai Phule Pune University for financial assistance.
References
- O’ Neill, L. A., Nat. Rev. Drug Discovery, 2006, 5, 549.
CrossRef - Cheeseright, T. J.; Holm, M.; Lehmann, F.; Luik, S.; Gottert, M.; Melville, J. L.; Laufer, S., J. Med. Chem., 2009, 52, 4200.
CrossRef - Vongtau, H. O.; Abbah, J.; Mosugu, O.; Chindo, B. A.; Ngazal, I. E.; Salawu, A. O.; Kwanashie, H. O.; Gamaniel, K. S., J. Ethnopharmacol.; 2004, 92, 317.
CrossRef - Chao, E. Y.; Caravella, J. A.; Watson, M. A.; Campobasso, N.; Ghisletti, S.; Billin, A. N., J. Med. Chem., 2008, 51, 5758.
CrossRef - Hynes, J. J.; Dyckmann, A. J.; Lin, S.; Wrobleski, S. T.; Hong, W.; Gillooly, K. M., J. Med. Chem., 2008, 41, 4.
CrossRef - Song, Y.; Connor, D. T.; Doubleday, R.; Sorenson, R. J.; Sercel, A. D.; Unangst, P. C.; Roth, B. D.; Gilbersten, R. B.; Chan, K.; Schrier, D. J.; Guglietta, A.; Bornemeier, D. A.; Dyer, R. D., J. Med. Chem., 1999, 42, 1151.
CrossRef - Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J., Phytochemistry, 2004, 65, 1389.
CrossRef - Cheng, J. H.; Hung, C. F.; Yang, S. C.; Wang, J. P.; Won, S. J.; Lin, C. N., Bioorg. Med. Chem., 2008, 16, 7270.
CrossRef - Lawrence, N. J.; Patterson, R. P.; Ooi, L. L.; Cook, D.; Ducki, S., Bioorg. Med. Chem. Lett., 2006, 16, 5844.
CrossRef - Vogel, S.; Ohmayer, S.; Burnner, G.; Heilmann, J., Bioorg. Med. Chem., 2008, 11, 4286.
CrossRef - Rojas, J.; Paya, M.; Dominguez J. N.; Ferrandiz, M. L., Bioorg. Med. Chem. Lett., 2002, 12, 1951.
CrossRef - Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Roiger, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C., J. Med. Chem., 1997, 40, 1347.
CrossRef - Deng, X.; Mani, N. S., Org. Lett., 2008, 10, 1307.
CrossRef - Smith, S. R.; Denhardt, G.; Terminelli, C., Eur. J. Pharmacol., 2001, 432, 107.
CrossRef - Baraldi, P. G.; Bovero, A.; Fruttarolo, F.; Romagnoli, R.; Tabrizi, M. A.; Preti, D.; Varani, K.; Borea, P. A.; Moorman, A. R., Bioorg. Med. Chem., 2003, 11, 4161.
CrossRef - Baraldi, P. G.; Manfredini, S.; Romagnoli, R.; Stevanato, L.; Zaid, A. N.; Manservigi, R., Nucleosides, Nucleotides Nucleic Acids, 1998, 17, 2165.
CrossRef - Prakash, O.; Kumar, R.; Prakash, V., Eur. J. Med. Chem., 2008, 43, 435.
CrossRef - Manfred R.; Michael, M.; Robert, S.; Wolfgang, G., Eur. Patent Appl. EP 334146:28, 1989.
- Hale, J. J.; Mills, S. G.; MacCross, M.; Dorn, C. P.; Finke, P. E.; Budhu, R. J., J. Med. Chem., 2000, 43, 1234.
CrossRef - Varma, R. S.; Prakash, C.; Prasad, R., J. Chem. Soc. Pak., 1986, 8, 117.
- Enyedy, I. J.; Sakamuri, S.; Zaman, W. A.; Johnson, K. M.; Wang, S., Bioorg. Med. Chem. Lett., 2003, 13, 513.
CrossRef - Tian, L.; Song, J.; Wang, J.; Liu, B., Chin. Chem. Lett., 2009, 20, 288.
CrossRef - Rangnekar, D.; Kanetkar, V., Indian J. Fibre Text. Res., 1990, 15, 132.
- Piorun, D.; Parusel, A.; Rechthaler, K.; Rotkiewicz, K.; Kohler, C., J. Photochem. Photobio. A Chem., 1999, 129, 33.
- Butler, T.; Patel, N.; Robert, M.; Baynes, N., PCT Int. Appl. WO, 2005019159, 2006.
- Gadhave, A. G.; Gaikar, R. B.; Kuchekar, S. R.; Karale, B. K., J. Heterocycl. Chem., 2014, 51, 1849.
CrossRef - Gadhave, A. G.; Gaikar, R. B.; Kuchekar, S. R.; Karale, B. K., Indian J. Chem., 2015, 54B, 383.
- Borhade, A. V.; Uphade, B. K.; Gadhave, A. G., Res. Chem. Int., 2015, 41, 1447.
CrossRef - Borhade, A. V.; Uphade, B. K.; Gadhave, A. G., Res. Chem. Int., 2016, 42, 6301.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









