Preparation of Pb(II)-Carboxymethyl Chitosan Pec PEGDE Film as Selective Asorbent for Removal Pb(II) Ion
Budi Hastuti1,2, Mudasir1, Dwi Siswanta1 and Triyono1
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Sekip Utara, Yogyakarta 55281, Indonesia.
2Department of Chemistry Education, Faculty of Faculty of Teacher Training and Education, Universitas Sebelas Maret, Jl. Ir. Sutami 36A Surakarta, 57126, Indonesia.
Corresponding Author E-mail : Bhastuti.uns@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330116
The aims of this research is to synthesize Pb(II)-Carboxymethyl Chitosan-Pectin crosslinked Polyethylene Glycol Diglisidyl Ether (Pb(II)-CCPecP) film by using templated ion of Pb2+ as selective adsorbent for Pb(II) ion. The structure of Pb(II)-CCPecP film adsorbent were characterized by FT-IR and scanning electron microscopy (SEM). The adsorption properties of Pb(II)-CCPecP film adsorbent in order to adsorp of Pb(II) ion in presence Cu(II) and Zn(II) were evaluated. FT-IR showed visible peak at 3441 and 1628 cm-1 indicate the film adsorbent have –COOH and -OH active group. The SEM of Pb(II)-CCPecP film show smoother surface and some holes and gaps are formed. The optimum condition for adsorption of Pb(II) ions by Pb(II)-CCPecP film adsorbent was occur at pH 5 and 200 min contacting time. The adsorption of Pb(II)-CCPecP film adsorbent toward Pb2+ was higher compared with Zn2+ and Cu2+ metal ion. The results revealed that the film was capable to removal of Pb(II) ion selectively in solution containing single metal ion or coexistence of three metals ion of Zn2+ and Cu2+.
KEYWORDS:carboxymethyl chitosan; pectin; PEGDE; Pb(II)-CCPecP film adsorbent; Pb(II)
Download this article as:| Copy the following to cite this article: Hastuti B, Mudasir M, Siswanta D, Triyono T. Preparation of Pb(II)-Carboxymethyl Chitosan Pec PEGDE Film as Selective Asorbent for Removal Pb(II) Ion . Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Hastuti B, Mudasir M, Siswanta D, Triyono T. Preparation of Pb(II)-Carboxymethyl Chitosan Pec PEGDE Film as Selective Asorbent for Removal Pb(II) Ion . Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=29865 |
Introduction
Chitosan, one of a cationic polysaccharida of glucosamine and N-acetyglucosamine from natural polymers. Chitosan derivative of chitin by deacetylated processes [1]. It is a nonpoisonous, biodegradable, bio compatible and bioactive materials. The amino and hydroxyl group of chitosan chain can serve as the chelation and reactions site, led to it able to form complexes with metal ion [2-4]. Chitosan is the most popular adsorbents for removal of metal ions, composed of poly (β–1,4)–2–amino–2–deoxy–D–glucopyranose. Chitosan and its derivative had reported able to act as a natur adsorbent in order to remove of metal ion from an aquoeos solution [5,6]. Chitosan as an heavy metal adsorbent is very high capability however its ability limited because of the weakness such as swelling, unsatisfying mechanical properties and solubility in acidic condition[7,8]. Chitosan modified by crosslinking[9], immobilizing into a matrix support [10] and blending[11] to form it stable on acid media and has good mechanical properties. To solve these problems, chemically crosslinked chitosan has be required. There are many crosslinking agent such as glutaraldehyde, epichlorohydrin, ethylene glycol diglycidyl ether, bis phenol A diglycidyl ether can be used to cross-link chitosan [12-16]. Reaction of chitosan with pectin able to make a stable polyelectrolyte complex that applied as an metal adsorbent [17].
Pectin is the middle lamella of polysaccarida and crosslinked with cellulose and hemicellulose fibers. The pectin is a polysaccharide composed of D-galacturonic acid units with α–(1,4) bonds, which constitute the “smooth regions”. Pectin has the main functional groups: hydroxyl, carboxyl, amide and methoxyl. These functional groups, especially the carboxyl groups, are potential for biosorption of heavy metal ion [18]. Heavy metal ions react with free carboxyl groups of pectin to form insoluble complexes [19].
Modified chitosan by conducted with pectin and crosslinked using Bis phenol A diglysidiyl ether (BADGE). Cross-linking between pectin and chitosan polymers form Chitosan-Pectin-BADGE able to improve the stability of adsorbent, particularly under low pH, and also to increasing of the adsorption capacity of the adsorbent[20]. Recently physical and chemical modification adsorbent lead to adsorption selectivity. Ion imprinting is a apropriate methode increase of adsorption selectivity. Metal ion templated technique combined with crosslinked chitosan which metal cation acts as template [8,21,22,23]. A lot of chitosan material template ion have been synthesized to adsorption convenient metal ion from aqueous solution, enhancing adsorption capacity. The selectivity of crosslinked template adsorbent is based on specification of ligand, coordination geometry, coordination number, charge and size of the metal ion [24,25].
In this work chloro acetate was grafting on chitosan to form carboxymethyl chitosan (CC), Polyethylene glicol diglycidyl ether (PEGDE) as crosslinked and pectin was synthesized to form Pb(II)-CCPecP by using Pb2+ as imprinting ion. The purpose of this work is prepare Pb(II)-CCPecP as a selectivity biosorbent toward Pb(II) metal ion.
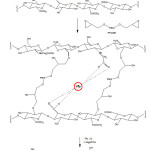 |
Figure 1: Synthesis Mechanism of Pb(II)–CCPecP adsorbent Click here to View Figure |
Experimental
Materials
Chitosan (Chi) deacetylation degree around of 75–85%, Pectin from apple peel (Pec) and Poly(ethylene glycol) Diglycidyl Ether (P) were purchased from Sigma–Aldrich (Germany). The others chemical i.e. chloroacetic acid, acetone, 37 % HCl, fumed HNO3, NaEDTA, NaOH, ethanol, isopropyl, metals standard solution (Pb, Cu, and Zn) were purchased from E. Merck (Germany).
Procedure
The Pb(II)–CCPecP adsorbent was prepared according to the previously reported procedure with slight modification [16]. In this work, the modification was carried out with replacing the crosslinking agent with Poly(ethylene glycol) Diglycidyl Ether (P). Characterization of the functional groups of the synthesized materials was conducted using Fourier Transform Infrared (FTIR) Spectrophotometer. .
Selectivity testing of an adsorbent done by contacting it with a solution containing a mixture of several metal ions in mole ratio Pb-Cu :1:1, 1:2, Pb-Zn 1:1, 1:2 and Pb:Cu:Zn=1: 1. The volume of the mixture was 10 ml. Analysis of the metal ion in a salution was determined using AAS.
Results and Discussion
FTIR spectrometer analysis was lead to predict the interaction between Pb (II) metal ions binding to the synthesized adsorbent (Fig. 1) that subsequently will be released to form Pb(II)-CCPecP adsorbent. In this work, Pb(II)-CCPecP adsorbent was synthesized through four reaction stages (as seen in Fig. 1). The synthesized step involves a) synthesis of CCPec film; b) imprinting of Pb(II) ion on CCPec film to form CCPec-Pb film; c) Cross-linked CCPec-Pb with PEGDE to form CCPecP bonding Pb(II); and d) releasing of Pb(II) ion from CCPecP to form Pb(II)-CCPecP film adsorbent. The result of FTIR spectra of chitosan at 3470 cm-1 indicated the vibration of the hydroxyl group. Meanwhile, the absorption at 1078 and 2930 cm-1 respectively corresponded to the CO– group and methylene group stretching vibration. The amide group (–NHCO) on the chitosan was observed at on wavenumber of 1634 cm-1).
Fig 1 showed the FTIR spectra of synthesized adsorbent compared to the raw material chitosan (Chi) (a) and pectin(Pec) (c). The CC adsorbent (b) showed a characteristic peak at 1600 and 1410 cm–1 to identify of the presence of a carboxylic group (–COOH). Furthermore, CC show absorption at a wavenumber of 3441 cm–1 that indicate of a –OH groups stretching vibration which is overlapping with stretching vibration of –NH group. FTIR spectra of pec.(c) are characteristically at 3380 cm-1 which indicated of the OH group vibration [19]. The absorption peak at 1750 cm-1 showed of C=O ester group [20]. Furthermore, the band at 1065 and 1628 cm-1 corresponded to –CO– group stretching vibration and–COOH group respectively.
The characteristic of active group of the adsorbent consist of typical active group CC, Pec and P. The characteristic peak of CC adsorbent cover of carboxylate groups and amine at wavelength 1660–1550 and 1762–1701 cm-1 cm-1 respectively. The peak adsorption of –COOH group at 1630–1600 cm-1 toidentify of Pec. While CCPecP synthesized, the absorption shows up peak at 1103 cm-1 which show the ether C–O stretch as well as the C–C stretch. Visible peak at 3402 to 3441 cm-1 on the graph a, b, and c show of –OH group vibration. The spectra at 2930cm-1 indicated of the –CH–CH vibration and –CH2 group. A new peak assigned to COO–Pb was observed at a wavelength of 1381 cm-1.
Interaction of functional group –COO– with Pb(II) ion on the adsorbent in stage b showed the weakening peak on1600 cm-1 wavelength. Characteristics of Pb(II)-CCPecP films was indicated by a peak at 1628 cm-1, which seen on stage b, and also appeared in stage c because of Pb(II) ion was separated from carboxylate group. In addition, the reappearance of –COO– ion indicated by a peak at 1450 cm-1.
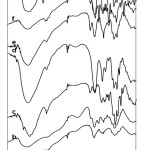 |
Figure 2: Chi (a), CC (b), Pec (c), CC-Pec (d), CCPec-Pb (e), CCPecP–Pb (f) and Pb(II) -CCPecP film adsorbent (g) Click here to View Figure |
Scanning electron microscope characterization
The structural morphology between pec, chi, CC and CMC-BADGE is futher supported by the difference in their SEM images. The SEM image of the surface of pec, chi, CC and Pb(II)-CCPecP are shown in Fig. 3. It show that the surface of the chi and CC smooth, fibrous and non porous appearance. The surface of pectin show a rugged structure distributed in the particles. While on the SEM of Pb(II)-CCPecP show smoother surface than the other, and hence some holes and gaps are formed, which are scattered around the surface and interior. The structure indicates that Pb(II)-CCPecP film may have a powerful adsorption capacity of Pb2+ arise from its unique structure and composition.
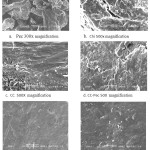 |
Figure 3: SEM image of a. Pectin, b. Chitosan; c. CC; d. CC-Pec; e. Pb(II)-CCPecP 250x, f. Pb(II)-CCPecP 2500x |
Contacting Time Adsorption Influence
The profile of Pb(II) adsorption by the contacting time effect shown in Fig. 3 Initially, Pb(II) was increased adsorpted (mg Pb(II)/g adsorbent) quickly due to most of active sites on the adsorbent were fully empty. Adsorption equilibrium was reached at 200 min adsorbent, and longer time no change in adsorption was occurred on CC and CCPecP, but on Pb(II)-CCPecP adsorbent absorption decreased, this happens because the ions Pb (II) which still inside of the cavity encounter desorption when Pb (II) contacted to the adsorbent.
Contact time effect and adsorption capacity of Pb(II) ion by CC, CCPecP and Pb(II)-CCPecP shown in Fig. 3
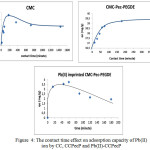 |
Figure 4: The contact time effect on adsorption capacity of Pb(II) ion by CC, CCPecP and Pb(II)-CCPecP Click here to View Figure |
pH Influence of adsorption
The pH media on adsorption of aqueous solution is one of an important factor on sorption of heavy metal ions which a great impact. The pH effect of the adsorbents are illustrated in Fig. 4. The structure of active group as a chelating agent can be changed by protonation at different pH values. The effect of pH media on pH 2, sorption of Pb(II) metal ions reduced up to a minimum. It happens because of the chelating groups bond the proton from Hidrogen. The optimal adsorption occur on pH 5, made functional group have negative charge and able to bind metal ions. In the higher pH, Pb(II) ions precipitated become Pb(OH)2 that can’t be adsorbed and therefore the adsorption ability decrease because adsorption process takes place simultaneously with the precipitation process.
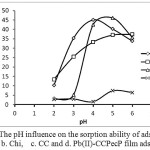 |
Figure 5: The pH influence on the sorption ability of adsorbent a. Pec, b. Chi, c. CC and d. Pb(II)-CCPecP film adsorbent. Click here to View Figure |
Adsorption Selectivity
The adsorption selectivity was determined by equilibrating 10 mg adsorbent with 10 mL of solution containing: Pb(II)/Cu(II), Pb(II)/Zn(II) and Pb(II)/Cu(II)/Zn(II) with each ion concentration of 0.5 mmol/L. The adsorption was carried out in batch system using magnetic stirrer at pH 5. Adsorption selectivity of Pb (II) by the adsorbent studied by the competition adsorption of Pb(II) in the presence of other ions. As a comparison used Cu(II) and Zn(II) ion. The mole ratio graph of the adsorption capacity and the percentage for the adsorbed Pb(II)-CCPecP toward of a solution containing of Pb/Cu metal ions are shown in Fig. 5 below.
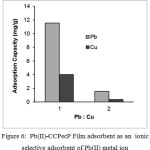 |
Figure 6: Pb(II)-CCPecP Film adsorbent as an ionic selective adsorbent of Pb(II) metal ion Click here to View Figure |
Selectivity of the adsorbent is influenced by the size of metal ions that have been imprinted in the adsorbent. Based on the HSAB (Hard and Soft acid and Base) principle by Pearson, carboxylate and amine groups are a hard base, which Pb(II), Zn(II) and Cu(II), is an intermediate acid (Borderline). Softness of acid can be determined by the parameters described by Dragon and Wayland. Acid softness (Y) decrease with the following order, Pb(II), Cu(II), Zn (II) and equal to 3.58; 2.89; and 2.34, respectively. Pb tends to be classified as a soft acid that has larger metal ions size, and Zn is more likely classified as hard acids which have a smaller metal ions size. The three metal ion size can be determined as follows: Pb2 +> Cu2+> Zn2+.
Selectivity of Pb(II)-CCPecP toward Pb(II) in the presence of Cu(II) ion
Figures 5 show adsorption capacity of Pb(II)-CCPecP film adsorbent which in absorb of metal Pb–Cu in mole ratio. The film adsorbent was potential as a selective adsorbent for Pb(II) metal ion in a mole ratio of Pb:Cu=1:1 with adsorption capacity adsorption of Pb(II) was 11.6 mg/g (26% Pb(II) adsorbed). At a mole ratio of Pb:Cu=1: 2 the adsorbent potentially as a selective adsorbent of Pb(II) with adsorption capacity of 4.0 mg/g and 10% Pb(II) adsorbed.
Pb(II)-CCPecP film is potential as a selective adsorbent toward Pb(II) ion in a mixture of mole ratio Pb–Cu 1:1 and 1: 2. The mold ion was constructed by releasing of Pb(II) metal ions using Na2EDTA so that adsorption of Pb(II) into the mold of adsorbent is more selective due to presence of its spesific cavity.
Selectivity of Pb(II)-CCPecP toward Pb(II) in the presence of Zn(II) ion
The graph of mole ratio of Pb(II) in presence of Zn(II) adsorbed by Pb(II)-CCPecP film versus adsorption capacity and the percentage ion are shown in Fig. 7.
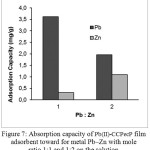 |
Figure 7: Absorption capacity of Pb(II)-CCPec Pfilm |
Fig. 7 shows the graph of adsorption capacity and Pb(II) adsorbed by the Pb(II)-CCPecP adsorbent film on a Pb(II)/Zn(II) solution. Pb(II)-CCPecP adsorbent films is potential as a selective adsorbent which adsorption capacity of Pb(II) in a mole ratio of 1:1 at 3.6 mg/g (13% of Pb(II) was adsorbed). The adsorption capacity of Pb(II) on the mix solution at a mole ratio Pb–Zn was1,9 mg/g and Pb(II) adsorbed by 8%; it is less selective incomparison with that on Pb(II)/Zn(II) solution.
Pb(II)-CCPecP adsorbent film is potential as a selective adsorbent in a mixture of Pb–Cu with a mole ratio of 1:1 and 1:2. The molds ion formed by desorption of metal ions Pb(II) using Na2EDTA, so that the mold will be more selective adsorption of Pb(II) because it has a large size with the same metal ion mold cavity is formed.
Selectivity of Pb(II)-CCPecP toward Pb(II) in the solution containing of Cu(II) and Zn(II)
The adsorption capacity and the percentage of Pb(II)-CCPecP in adsorption Pb(II) in the solution that presense of Cu(II) and Zn(II) are shown in Figure 7:
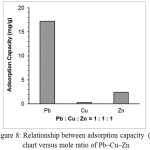 |
Figure 8: Relationship between adsorption capacity (b) chart versus mole ratio of Pb–Cu–Zn |
Fig. 8 shows adsorption capacity Pb(II)-CCPecP film adsorbent toward Pb(II) in Pb(II)/Cu(II)/Zn(II) multimetal solution. Pb(II)-CCPecP film adsorbent was potential as a selective adsorbent for Pb(II) in a multimetal solution of Pb(II)/ /Zn(II) Cu(II) with 17.22 mg/g adsorption capacity and 50% Pb(II) adsorbed. Pb(II)-CCPecP films adsorbent was the most selective adsorbents to adsorb Pb(II) in a solution mixture containing all of three metals Pb–Zn–Cu because Pb(II) imprinted polymers created pores that appropriate to the size of Pb(II) hydrated ion. Pb(II) hydrated ion has bigger ionic radii than hydrated Cu(II) and Zn(II) ions. The ionic radii of hydrated Pb(II), Cu(II) and Zn(II) are 2.74, 2.07 and 0.74 Å,respectively [17]. The hydrated metal ion smaller than hydrated Pb(II) ion can enter to the cavity and be adsorbed on the adsorbent.
Conclusion
In this work Pb(II)-CCPecP film adsorbent was formed into Pb(II) templated polymer by a template forming process using Pb(II) metal ions. The Pb(II) ions corporated in the adsorbent, was released using Na2EDTA solution to make Pb(II)-CCPecP film adsorbent. The active group contained in the Pb(II)-CCPecP is a -OH, –COOH and –COO–. The Pb(II)-CCPecP film adsorbent was a selective adsorbent of Pb(II) ions in the presence of Cu(II) and Zn(II) in solution. Interaction between Pb(II) and Pb(II)-CCPecP was dominantly by chelation process. The Pb(II)-CCPecP adsorbent has a good potential to remove Pb2+ ion in solution containing single metal ion or coexistence of three metals ion of Zn2+ and Cu2+
Acknowledgement
This research was funded by the Competitive Research Grants Program of the Directorate of Higher Education of the Republic of Indonesia (DP2M DIKTI, 2015).
References
- Ge, H.C., Wang, S.K. Carbohydr. Polym. 2014. 113, 296–303.
CrossRef - Jiang, W., Chen, X.B., Pan, B.C., Zhang, Q.X. Teng, L., Chen, Y.F., Liu, L. J. Hazard. Mater. 2014, 276,295–301.
CrossRef - Wu, F.C., Tseng, R.L., Juang, R.S. J.Environ. Manage. 2010, 91,798–806.
CrossRef - Trujillo-Reyes, J., Peralta-Videa & J.R., Gardea-Torresdey, J.L.. J. of Hazard. Mater. 2014, 280, 487–503.
CrossRef - Ge, H.C., Ma, Z.W, Carbohydr.Polym. 2015, 131, 280–287.
CrossRef - Yong, S.K., Bolan, N.S., Lombi, E., Skinner, W., Guibal, E.. Critical Reviews in Environm Sci and Tech, 2013, 43(16),1741-1794.
CrossRef - Farag, S., Kareem, S.S.A. Carbohydr.Polym. 2009, 78 (2): 263–267.
CrossRef - Huacai, G., Tingting, H., Xiaodong, C, J. of Hazard. Mater. 2016, 308, 225–232.
CrossRef - Suguna, M., Kumar, S.S., Reddy, A.S., Boddu, V.M., Krishnaiah, A. Can. J. Chem.Eng., 2011, 89(4), 833–843.
CrossRef - Copello, G.J., Varela, F., Vivot, M., Diaz, L.E. Bioresour. Technol. 2008, 99(14), 6538–6544.
CrossRef - Zhu, H.Y., Fu, Y.Q., Jiang, R., Yao, J., Xiao, L., Zeng, G.M. Bioresour. Technol., 2012, 105,24–30.
CrossRef - Ge, H.C., Huang, S.Y. J. Appl. Polym. Sci. 2010, 115,514–519.
CrossRef - Hsien, K.J, Futalan, C.M., Tsai, W.C., Kan, C.C., Kung, C.S., Shen, Y.H., Wan, M.W. Desalin. Water Treat. 2013, 51, 5574–5582.
CrossRef - Bal, A., Ozkahraman, B., Acar, I., Ozyurek, M., Guclu, G. Desalin. Water Treat. 2014., 52:3246–3255.
CrossRef - Boamah, P.O., Huang, Y., Hua, M.Q., Zhang, Q., Liu, Y.Y., Onumah, J., Wang, W., Song, Y.X. Carbohydr. Polym. 2015, 122, 255–264.
CrossRef - Monier, M., Ayad, D.M., Abdel-Latif, D.A. Colloids Surf. 2012. 94, 250–258.
CrossRef - Hastuti, B., Mudasir, Siswanta, D., Triyono. Int. J. of Chem. Eng. and App., 2013.4 (6),349-353.
- Mata, Y.N., Blázquez, M.L. Ballester, A., González, F., Mu˜noz, J.A. Chem. Eng. J. 2009, 150, 289–301.
- Kupchik, L. A., Kartel’ N. T., Bogdanov, E. S., Bogdanova, O. V., Kupchik, M. P. Russian J.l of App. Chem, 2006., 79,(3), 457–460.
- Hastuti, B., Siswanta, D., Mudasir, Triyono., Indones. J. of Chem, 2015, 15 (3), 248 – 255.
CrossRef - Hande, P.E., Samui, A.B., Kulkarni, P.S. Environ. Sci. Pollut. Res. 2015, 22, 7375–7404.
CrossRef - Zhang, M., Helleur, R., Zhang, Y., Carbohydr. Polym Carbohydr. Polym. 2015, 130, 206–212.
CrossRef - He, J., Lu, Y.C., Luo, G.S.. Chem Eng J, 2014, 244, 202–208.
CrossRef - Fan, L.L., Luo, C.N. , Zhen, L. , Lu, F.G., Qiu, H.M.. J.Hazard. Mater. 2011, 194, 193–201.
CrossRef - Chen, C.Y., Yang C.Y., Chen, A.H. J.Environ. Manage. 2011, 92, 796–802.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









