Oligomers Solidification Depending on the Nature, Molecular Mass, Type and Reactional Group Containing
Vasiliyp. Medvedev1, Marata. Vaniyev1, Saulea. Sakibayeva2 and Anastassiya Y. Kovaleva2*
1Volgograd State Technical University, Russian Federation, 400005, Volgograd, Lenin av., 28.
2Auezov South Kazakhstan State University, Kazakhstan, 160012, Shymkent, Tauke Khan av., 5.
Corresponding Author E-mail: anastasiya2301@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/330131
The article is devoted to regularity defining of curing oligomers, and prepolymers with hydroxyl and isocyanate groups and double bonds reactive oligomers.The features of the spatial structure of elasticpolyurethane based oligomers and prepolymers with the definition of the physical and chemical bonds share, as well as the chain interval length between grid points were researched. The possibility of oligodiendiolecuring in the presence of a methacrylate component on the mechanism of radical polymerization was experimentally confirmed.To achieve the objectives rheokinetic method of analysis (rotational viscometer), thermometric and iodometric methods, IR spectroscopy, differential scanning calorimetrywere used.
KEYWORDS:oligodiendiole; polyisocyanate; chain branching agent; polydieneurethane; coatings
Download this article as:| Copy the following to cite this article: Medvedev V, Vaniyev M, Sakibayeva S, Kovaleva A. Y. Oligomers Solidification Depending on the Nature, Molecular Mass, Type and Reactional Group Containing. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Medvedev V, Vaniyev M, Sakibayeva S, Kovaleva A. Y. Oligomers Solidification Depending on the Nature, Molecular Mass, Type and Reactional Group Containing. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=28093 |
Introduction
Currently urethane forming materials and compositions based on them are in demand for the production of sealants, glues, adhesives, waterproofing, sport, and other types of roof coverings.The main range of reactive oligomers of oligodiene nature for such compositions is based on hydroxyl-containing oligomers PDI-1K brand,Krasol LBH 3000 rubbers and PolyBDof European production. Oligomeric rubber PDI-1K is characterized by aninsufficientfunctionality, a relatively high proportion of non-functional and multi-functional molecules, which leads to defects in the vulcanization of elastic polyurethane grid and adversely affects to the elastic-strength properties[1].
From the viewpoint of improving the structure of the elastic polyurethane spatial grid and improve the properties of the oligomeric rubber materials and compositions on their basis their use is reasonable not only for curing the terminal hydroxyl groups of the oligomer, but also double bonds in macromolecules chain. Thus isocyanate chain extending agents for urethane scheme and acrylate compounds for crosslinking macromolecular chains elongated at double bonds can be used. There is reason to anticipate a significant improvement in the properties of complex materials obtained as a result of the combined curing method [2-3].
Objective of this research phase consists, firstly, in the study of the curing process according to the nature and molecular weight of oligomers; secondly, in the research of the influence of type and content of reactive groups, the type and amount of hardener, elongation and branching agents to process urethane curing mechanism; thirdly, in the research capabilities and features curing oligodiendioles by polymerization mechanism in the presence of methacrylate containing compounds [4,5].
These aspects are little-investigatedand hardly covered in the scientific and technical information sources, which determines the scientific novelty of the work. Scientific research novelty is also concluded in the fact that oligodiendioles curing by combined method involving double bonds and terminal hydroxyl groups in the presence of an acrylate crosslinker component and a peroxide initiator in combination with an activator of its decay was proposed for the first time.
Material And Method
For curing compositions based on low molecular weight rubbers PDI-1K and Krasol LBH-3000 polyisocyanate curing agentwas used. It is a mixture consisting of 4,4′-methylene diphenylisocyanate in an amount of 81.802%, its isomer 1-isocyanate-2- (4-isocyanatebenzyl) benzene in an amount of 16.170% and a homolog 1-isocyanate-2,4-bis (4-isocyanatebenzyl) benzene in an amount of 2.028%. Mass fraction of isocyanate groups is in the range of 30-33%. Viscosity at 25˚Сis 134 MPa·s. Ethacure 300 (a mixture of isomers of 3,5-dimethylthio-2,6-toluenediamine and 3,5-dimetildio-2,4-toluene diamine), and glycerolwerehardeners for prepolymers.The standard methods of the research were used.
Results And Discussion
As a first step of the research the features of oligomers hardening by the method of rotational viscometry were studied. The basic methodology has been previously worked with compositions based on hydroxyl reactive oligomer mark PDI-1K, characterized by a molecular weight of 3,000.The ratio of isocyanate groups to the sum of the hydroxyl (oligomer of glycerol) at a fixed dose of chain brunching agent and urethane catalyst was varied. Formulations are shown in table 1.
Table 1: Formulations of compositions based on reactive oligomer PDI-1K
|
RatioNCO / SОН), mole / mole |
0,75 |
0,9 |
1,0 |
1,25 |
1,5 |
1,75 |
2,0 |
| Ingredients of compositions |
Ingredients’content, mass.h. |
||||||
| PDI-1K |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
| Polyisocyanate |
12,1 |
14,6 |
16,2 |
20,3 |
24,3 |
28,4 |
32,4 |
| Glycerol |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
| Tin dibutildilaurinate |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
0,02 |
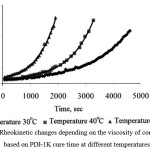 |
Figure 1: Rheokinetic changes depending on the viscosity of compositions based on PDI-1K cure time at different temperatures |
According to breaking of viscosity dependence from time in Fig. 1 it is possible to define the effect of temperature on the nature of the curing. In particular, increasing the curing temperature from 300C to 500C leads to an increase in the main phase reaction of urethane in three times (in this case, the ratio of NCO / S OH = 1.25).
In table 2 numerical values of the constants curing rate are given.
Table 2: Curing rate constant values depending on the temperature for compositions on the basis of PDI-1Kat a ratio NCO / S OH = 1.25
|
Temperature, К |
Constant curing rate, (Кh) с-1 |
1/Т, К-1 |
ln(Кh) |
|
303 |
0,00080 |
3,30Е-03 |
-7,1309 |
|
313 |
0,00130 |
3,19Е-03 |
-6,6454 |
|
323 |
0,00160 |
3,10Е-03 |
-6,4378 |
On the basis of received values the dependences in coordinates were defined lnK = f (1 / T) (figure 2).
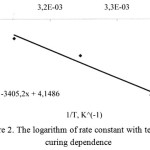 |
Figure 2: The logarithm of rate constant with temperature curing dependence |
Also the dependence of activation energy from ratio of reactive groups (table 3) and the dependence of the gelation time of the curing temperature and the ratio of reactive groups (table 4) were defined.
Table 3: Dependence of activation energy from ratio of reactive groups.
|
RatioNCO / SOH, (mole / mole) |
Activation energy, (kJ / mole) |
|
1,25 |
28,3 |
|
1,5 |
27,6 |
|
2,0 |
26,9 |
Table 4: Dependence of the gelation time of the curing temperature and the ratio of reactive groups
|
RatioNCO / SОН), (mole / mole) |
Temperature, °С |
Gelation time, sec |
|
1,25 |
30 |
3170 |
|
40 |
2025 |
|
|
50 |
1268 |
|
|
1,5 |
30 |
2483 |
|
40 |
1638 |
|
|
50 |
1137 |
|
|
2,0 |
30 |
1032 |
|
40 |
784 |
|
|
50 |
585 |
Thus, as a result of research for identifying the characteristics of the curing compositions based on reactive PDI-1K oligomer, it was found that an increase in the content of the polyisocyanate in studied temperature range is an increase in the values of the rate constants increase viscosity (K) with a slight decrease in the activation energy of viscous flow.
With the increase of proportion of NCO / SОН, in range of 1.25 – 2.0, gelling time decreases. Considering that in the cured elastic polyurethanes besides chemical transverse bonds it is possible to create intermolecular bonds, it is important to properly choose a solvent for detection of special polyurethane net with the Klaff-Gleding method. Toluene is the solvent which has the most optimal thermodynamic relation to investigated polyurethanes. It was discovered that material sveling in toluene is reversible. Spectral investigation showed no relation between solvent and reactive centers of polyurethane.
Investigation of structural particularities as a result of interreaction of isocyanate and hydroxyl groups on the example of Krasol LBH based cured compositions made it possible to determine that the increase percentage of curing component (polyisocyanate) in composition will bring to growth of general, physical and chemical bonds. (figure 3.10). This fact can be explained by developing of more crosslinked spatial structure due to increase of tough urethane blocks. It is noticed that curves which depict dependence of Мс for NCO / SОН have decreasing tendency. It is important that with the increase of molecular mass of oligomers Мсalso increases (figure 3.11). It tells about less crosslink density.
Conclusion
There was an investigation of structural particularities of elastic polyurethanes, which are formed by curing of oligomer Krasol LBH based compositions, as a result of interreaction of isocyanate and hydroxyl groups. It was observed that increase of curing component leads to growth of general, physical and chemical bonds. It is explained by the developing of the more crosslinked spatial structure due to increase of tough urethane blocks. It is detected that Мс(length of chain section between joints of spatial connections net) decrease in case of excess of isocyanate groups relating to hydroxyl groups. An important observations is that Мс increases with the increase of molecular mass of oligomers. In other words it tells about less crosslink density of elastic polyurethanes, which has an effect on elastic properties and hardness.
References
- Ukrainskaya,S.I.; Medvedev,V.P.;Chapurkin,V.V. Development of receipts and research of the compositions on the base of new hydroxyl containing oligomer rubber. Proceedings of Volgograd State Technical University. Chemistry and technology of elementorganic monomers and polymer materials. 2012,7,154- 159
- Medvedev,V.P.;Ukrainskaya,S.I.;Chapurkin,V.V.; Murzin,A.V.Polyurethane elastomers on the base of oligobutandienediol with 1,2-structure of double bonds.Caoutchouc and rubber.2012, 1,2-5.
- Medvedev,V.P.;Ukrainskaya,S.I.;Chapurkin,V.V.;Murzin,A.V. Polyurethane elastomers on the base of NISSO РВ G3000 oligobutandienediol. Glues. Sealants. Technologies. 2012, 12,11-15.
- Sergeyeva, L.M.; Lipatove, Y.S.; Binkevich, N.I.. Research of the density of spacing grid and nature of cross-linking bonds in polyurethane elastomers // In book. Synthesis and Physics and Chemistry of polyurethane.1967, 199
- Medvedev, V.P.; Vaniyev, M.A.; Sakibayeva, S.A.; Kovaleva, A.Y. Operating Characteristics of Diene-Urethane Elastomers on the base of NISSOG 3000 Oligodienediol. Oriental Journal of Chemistry. 2016, 5, 2363-2370.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









