Antioxidant Activity of Extracts of HalodulepinifoliaSeagrass from Solvents with Different Polarities
Ace Baehaki, Indah Widiastuti, Herpandi and Nurul Jannah
Study Program of Fisheries Product Technology, Faculty of Agriculture, Sriwijaya University, Indralaya, South Sumatera, Indonesia.
Corresponding Author E-mail: ace76_none@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330120
The purpose of this study was to analyze phytochemistry contents and antioxidant activity ofextracts from seagrass of Halodulepinifoliafrom solvents with different polarities. Parameters of research werephytochemical content, DPPH scavenging activity and reducing power. The result showed content of phytochemical compounds of ethanol extract seagrass were flavonoids, tannins, saponins, steriods andtriterpenoids. The use of ethyl acetate solvent showed phytochemical compounds were flavonoids, steroids and triterpenoids. For n-hexane solvent showed phytochemical compounds were steroids and triterpenoids. The highest of antioxidant activity with DPPH method (IC50) of H.pinifolia was 18.7 ppm with ethanol extract. The highest of reducing power of H.pinifolia was 1.749.
KEYWORDS:Seagrass; Halodulepinifolia; antioxidant
Download this article as:| Copy the following to cite this article: Baehaki A, Widiastuti I, Herpandi H, Jannah N. Antioxidant Activity of Extracts of HalodulepinifoliaSeagrass from Solvents with Different Polarities. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Baehaki A, Widiastuti I, Herpandi H, Jannah N. Antioxidant Activity of Extracts of HalodulepinifoliaSeagrass from Solvents with Different Polarities. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=29520 |
Introduction
Seagrasses are flowering plants (angiosperms) which grow in marine, fully saline environments.Seagrasses are a rich source of structurally novel and biologically active metabolites which they produce inorder to sustain the extreme environmental conditions prevailing under sea1
Seagrasses produce antioxidant compounds that inhibits the oxidation of other molecules and there are many reports describing antioxidant activities2-5, antifungal6, antiviral7, anti-inflammatory8, antidiabetic9and antibacterial10-12. However reports on the phytochemical constituents of seagrasses and their bioactive activity of Indonesian sea are limited with the exception of few studiesin this research13,14, we reported thatantioxidant activity of extracts of Halodulepinifoliaseagrass from solvents with different polarities.
Materials and Method
Preparation of Seagrass extract
Extraction of Halodulepinifoliaseagrassby stratified maceration method using n-hexane, ethyl acetate and ethanol. Sea grass powder were soaked in 2 L with solvent (1:4 w/v), and kept for 2 x 24 h in a shaker. The solution is filtered using the number 42 Whatman filter paper to obtain the filtrate. The filtrate is dried using a freeze dryer to remove the solvent that may remain in the extract
Phytochemical Screening of Halodulepinifolia
Test of flavonoids, alkaloids, saponin, steroids, triterpenoidswere determined by Harborne method15.
DPPH radical scavenging activity
DPPH radical scavenging activity was measured based on methods described in Hananiet al.16.
Reducing power
Reducing power was determined by Oyaiza method17.
Result and Discussion
The phytochemical screening
As seen as Table 1 showed content of phytochemical compounds of extract of seagrass were flavonoids, alkaloids, tannins, saponins, steroids and triterpenoids.
Table 1: Phytochemical compound ofextract of H. pinifoliaseagrass
|
Sample |
Parameter |
Result |
||
|
n-hexane |
Flavonoids |
Negative |
||
| Alkaloids | Wegner |
Negative |
||
| Mayer |
Negative |
|||
| Dragendorf |
Negative |
|||
| Tannins |
Negative |
|||
| Saponins |
Negative |
|||
| Steroids |
Positive |
|||
| Triterpenoids |
Positive |
|||
|
Ethyl acetate |
Flavonoids |
Positive |
||
| Alkaloids | Wegner |
Negative |
||
| Mayer |
Negative |
|||
| Dragendorf |
Negative |
|||
| Tannins |
Positive |
|||
| Saponins |
Negative |
|||
| Steroids |
Positive |
|||
| Triterpenoids |
Positive |
|||
|
Ethanol |
Flavonoids |
Positive |
||
| Alkaloids | Wegner |
Negative |
||
| Mayer |
Negative |
|||
| Dragendorf |
Negative |
|||
| Tannins |
Positive |
|||
| Saponins |
Positive |
|||
| Steroids |
Positive |
|||
| Triterpenoids |
Positive |
|||
The result showed content of phytochemical compounds of ethanol extract seagrass were flavonoids, tannins, saponins, steriods andtriterpenoids. The use of ethyl acetate solvent showed the phytochemical compounds were flavonoids, steroids and triterpenoids. For n-hexane solvent showed phytochemical compounds were steroids and triterpenoids.
DPPH radical scavenging activity
Method of DPPHradical scavenging activity is very popular for the research of natural antioxidants18. The extraction with solvents of increasing polarity involves of separating compounds of a plant according to their degree of solubility. DPPH radical scavenging activity of hexane, ethyl acetate and ethanol extracts obtained of the Halodulepinifolia were shown in Figure 1. The maximum DPPH radical scavenging activity was recorded in ethanol extracts followed by ethyl acetate and n-hexane.
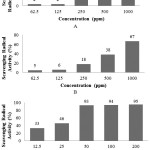 |
Figure 1: Scavenging radical DPPH activity extract of H.pinifoliaSeagrass (A= n-hexane, B=ethyl acetic and C= ethanol) |
The IC50 of extract was 18.7 ppm for ethanol extract, 696.2 ppm for ethyl acetate extract and 2,378.2 ppm for n-hexane extract. The IC50 value for vitamin C was 7.7 ppm (Figure 2). The results indicate that the antioxidant activity of the methanol extract of H.pinifoliaseagrass is higher than that of ethyl acetate and n-hexane extracts. Antioxidant activity of extractHalodulepinifolia could be due to their phytochemical compounds. The phytochemical compounds present in the extract, which are responsible for this activity. The phytochemical tests indicated the presence of flavonoids, tannins, saponins, steriods and triterpenoids in the crude methanolic extract.
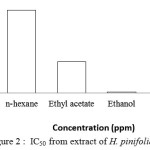 |
Figure 2: IC50 from extract of H.pinifoliaSeagrass |
Reducing power
Reducing power of extract of H.pinifoliadepicted in Figure 2.Increasing of concentration of H. pinifoliaindicates an increase in reducing power
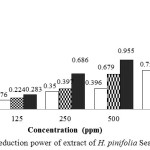 |
Figure 3: Reduction power of extract of H.pinifoliaSeagrass |
The reducing power is considered as a significant indicator of potential antioxidant activity of compound or sample. A potential antioxidant willreduce the ferric ion to the ferrous ion. Reducing power of extract of H. pinifoliais probably due to the presence of phytochemical compounds that can serve as an electron donor.
References
- Neelima CSS, Seenivasan R. 2015. DPPH radical scavenging activity of selected Seagrasses from South East Coast of India. Int. J Adv. Res, 2015, 3, 950 – 956.
- Athiperumalsamy T, Devi Rajeswari V, HasthaPoorna S, Kumar V and Louis Jesudass L. Antioxidant activity of seagrasses and seaweeds. Botanica Marina, 2010, 53, 251–257.
- Ragupathi Raja Kannan R, Arumugam R and Anantharaman P. In-vitro antioxidant activities of Enhalusacoroides.Asian Pacific J Tropical Med, 2010, 11, 898–901.
- RagupathiRagupathi Raja Kannan R, Arumugam R, Grignon Dubois M and Anantharaman P. Antioxidant activity of seagrasses of the Mandapam coast, India. Pharma.Biol,2012, 50, 182–187.
CrossRef - Ragupathi Raja Kannan R, Arumugam R, MeenakshiS andAnantharaman P. Thin layer chromatography analysis of antioxidant constituents of seagrasses of Gulf of Mannar. Int J. ChemTech Res, 2010, 2, 1526–1530.
- Arumugam R., Ragupathi Raja Kannan R, Arivuselvan N and Anantharaman P. Antimicrobial potential of some seagrasses against phytopathogens. Seaweed Res. Utilization,2010, 32, 177–183.
- Rowley DC, Hansen MST, Rhodes D, Sotriffer CA, Ni H, McCammon JA, et al. Thalassiolins A–C: New marine – Derived inhibitors of HIV cDNAintegrase. Bioorganic Med. Chem,2002, 10, 3619–3625
CrossRef - Hua KF, Hsu HY, Su YC, Lin IF, Yang SS, Chen YM, et al. Study on the anti-inflammatory activity of methanol extract from seagrassZostera japonica. J. Agric. Food Chem, 2006, 54, 306–311.
CrossRef - Gokce G and Haznedaroglu MZ. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidoniaoceanica extract. J. Ethnopharma, 2008, 115, 122–130
CrossRef - Ragupathi Raja Kannan R, Arumugam R, and Anantharaman P. Antibacterial potential of three seagrasses against human pathogens. Asian Pacific J, Tropical Med, 2010, 11, 890–893.
CrossRef - Ragupathi Raja Kannan R, Arumugam R and Anantharaman P. Chemical composition and antibacterial activity of Indian seagrasses against urinary tract pathogens. Food Chem,2012, 135, 2470–2473.
CrossRef - Sreenath Kumar C, Sarada DVL, Gideon TP and Rengasamy R. Antibacterial activity of three South Indian seagrasses, Cymodoceaserrulata, Halophilaovalis and Zosteracapensis. World J. Microbiol.Biotechnol, 2008, 24, 1989–1992.
CrossRef - Baehaki A, Supriadi A and Pratama, MC. Antioxidant Activity of Methanol Extract of HaloduleuninervisSeagrass from the Coastal of Lampung, Indonesia.Res. J.Pharma. Biol. ChemSci,2016, 7, 173-177.
- Supriadi A, Baehaki A and Pratama, MC. Antibacterial Activity of Methanol Extract from Seagrass ofHaloduleUninervisin the Coastal of Lampung, 2016, 8, 77-79
- Harborne JB. 1987. Phytochemical Methods2nd edition. Chapman and Hall, New York.
- Hanani E, Moneim B, Sekarini R. Identification of antioxidant compounds in the sponge Callispongiasp of the Thousand Islands.Magazine Pharm. Sci2005, 2, 127-133.
- Oyaizu, M..Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine.Japanese JNutr,1986, 44, 307–315.
CrossRef - Villano D, Fernandez-Pachon MS, Moya ML, Troncoso AM, Garcia- Parilla MC. Radical scavenging ability of phenolic compounds towards DPPH free radical. Talanta,2007,71,230–235.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









