Study on Adsorption of Malachite Green by Date Palm Fiber
Mashael Alshabanat*, Rasmiah S. Al-Mufarij and Ghadah M. Al-Senani
Chemistry Department, Science College, Princess Nourahbint Abdulrahman University, Riyadh, KSA.
Corresponding Author E-mail: ma.naif@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/320636
In this study, the adsorption of malachite green (MG) onto date palm fibers (DPF) was examined in aqueous solutions. The Langmuir and Freundlich adsorption isotherm models were employed to describe the equilibrium adsorption data. The Freundlich isotherm model was found to fit the adsorption data The removal percentage of MG dye from aqueous solutions via adsorption onto DPF adsorbent varied with pH and temperature, showing that these factors play a key role in the adsorption process. The removal percentage was found to increase with decrease in the solution temperature and in alkalinemedia. The adsorption kinetic data were fitted with a pseudo-second-order model.
KEYWORDS:Malachite green; Date palm fibers; Percentage removal; Adsorption
Download this article as:| Copy the following to cite this article: Alshabanat M, Al-Mufarij R. S, Al-Senani G. M.. Study on Adsorption of Malachite Green by Date Palm Fiber. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Alshabanat M, Al-Mufarij R. S, Al-Senani G. M.. Study on Adsorption of Malachite Green by Date Palm Fiber. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=26391 |
Introduction
Color removal from industrial effluents hasbeen a subject of investigation because of its potential toxicity. Malachite green (MG) is one of the dyes that is extensively used in the textile industry. It exhibits toxic properties that are known to cause carcinogenesis, mutagenesis, teratogenesis and respiratory toxicity[1, 2]. In recent times, adsorption-techniques for wastewater-treatment have become very popular because of to their improved efficiency in the removal of pollutants too stable for biological methods[3]. Research in the past few years has been focused on using agricultural wastes as adsorbent materials because they are inexpensive, eco-friendly and renewable. For instance, pumpkin seeds [4], the husk of mango seeds[5], cotton plant[6], coconut bunch wastes[7], orange peels[8], and pineapple stem [9] have been studied as potential adsorbents. Date palm is an important fruit crop in Saudi Arabia as well as around the world. It is estimated that the annual date palm agricultural wastes comprise more than 20 kg of dry leaves and fibers for each date palm tree [10]. Thus, utilizing the date palm waste as a potential adsorbent for wastewater treatment via adsorption is of considerable interest. In this study, the adsorption of MG from aqueous solutions was studied using the brownish fibrous sheath that surrounds the trunk of the date palm (known as leef) as an adsorbent. In addition, we studied the process kinetics. The removal percentage was calculated to determine the influence of pH and temperature on the adsorption.
Materials and Method
The date palm fibers (DPF)wastes were obtained a local from a farm in the southern region of Riyadh city in Saudi Arabia. They were cut, ground and sieved to asize ≤63 μm. A typical date palm fiber can be seen in Fig. 1[10]. MG was received from Techno Pharmchem (India)was used as an adsorbate.The dye content is at least 90%.Test solutionsof desired concentrations are prepared from the stock solution using distilled water.
Adsorption tests were carried out by using batch experiments. For each test, 0.5 g of the adsorbent was placed in screw-capped Erlenmeyer flasks containing 25 mL of MG solution. The flasks were shaken constantly for a sufficient period to achieve equilibrium using an orbital shaker (J.P. Selecta, Spain)at 100rpm and 25°C. Then the solution was filtered using Whatman filter paper (125 mm Ø, cat. No. 1001 125).The dye uptake was monitored with a spectrophotometer (Jenway model 6800 UV/VIS) by measuring the absorbance at λmax = 616 nm. The adsorption at the equilibrium,qe (mol/g), was calculated using the following equation:
![]()
where (mol/L) are the initial and the equilibrium liquid-phase concentrations of the dye, respectively. V is the volume of the solution (in liters), and m is the mass of the dry adsorbent (in grams). The equilibrium adsorption data were fitted with two isotherm models, the Langmuir and Freundlich models.
The effect of pH was studied by preparing suitable adsorbent–adsorbate solutions at room temperature with fixed adsorbent dosage and initial dye concentration at different pH.The pH was adjusted by adding NaOH (1 M) or HCl (1 M) solutions. The temperature effect on the adsorption process was studied by varying the adsorption temperature with a constant initial concentration of the solution. The removal percentage was calculated using Eq. (2).
Removal (%) = [ (Co – Ce) /Co ] × 100 (2)
The kinetic study was also performed at room temperature with a flask that was identical to the one used for batch equilibrium tests. The flask was shaken constantly to achieve equilibrium and the aqueous samples were collected at preset-time intervals,and concentrations of MG were measuresed.
![Figure 1: Date palm fi bers( a) on the tree, ( b ) separated [10]](http://www.orientjchem.org/wp-content/uploads/2016/12/Vol32No6_Stud_MASH_fig1-150x150.jpg) |
Figure 1: Date palm fi bers( a) on the tree, ( b ) separated [10] |
Results and Discussion
Adsorption Study
Adsorption isotherms indicate how adsorbates are distributed between the liquid phase and the solid phase when the adsorption process reaches equilibrium [11]. The adsorption isotherm is important for understanding how the adsorbate interacts with the adsorbent, giveingan idea of the adsorption capacity of the adsorbent [12]. Fig. 2 illustrates the adsorption isotherm of MG onto DPF. The maximum experimental adsorption capacity for the dye onto DPF is approximately 0.2136 mol g−1. From the figure, we can be seethat the adsorption isotherm is an Stype (based on Giles et al. classification), ndicating that the adsorption becomes easier as the concentration increases[13]. In practice, the S curve of the adsorption isotherm indicates a strong competition between the solvent and the adsorbed species for the adsorbent surface sites [13]. Analyzing the isotherm data by fitting it to different isotherm models is important for identifying a suitable model that can be used for design purposes [14]. The adsorption isotherm models of Langmuir (Eq. (3)) and Freundlich (Eq. (4)) were applied to describe the sorption data. The applicability of the isotherm equation to describe the adsorption process was judged in terms of the correlation coefficients(R2)and the maximum adsorption capacities.
![]()
where Kf is the equilibrium concentration, is the amount of adsorbate adsorbed per unit mass of adsorbent (in mol/g), and Qₒ and b are the Langmuir constants related to the adsorption capacity and rate of adsorption, respectively. In the Langmuir model, a plot of 1/Ce against 1/qe yields a straight line with a slope of (1/bQₒ) and an intercept of 1/Qₒ, as shown in Fig. 3.
![]()
where Kf and n are Freundlich constants related to the adsorption capacity and adsorption intensity, respectively. The n value indicates the degree of nonlinearity between solution concentration and adsorption as follows: if n=1, then the adsorption is linear;if n˃1, then the adsorption is a physical process; and if n˂1, then the adsorption is a chemical process[15].In the Freundlich model, a plot of log qe against log Ce yields a straight line with a slope of 1/n and an intercept of log Kf, as shown Fig. 4. The slope 1/n ranging between 0 and 1 is a measure of adsorption intensity or surface heterogeneity[16]. It becomes more heterogeneous as its value gets closer to zero; a value of 1/n below one indicates a normal Langmuir isotherm while that above one is indicative of cooperative adsorption[16].
The constants in these models, i.e.,Qₒ , b, Kf, and n, and the correlation coefficients,R2 are given in Table 1. According to the R2 values, both models seemed to represent the equilibrium adsorption data. However, Langmuir isotherm is difficult to interpret because it is characterized with a negative qe value.Therefore, the adsorption of MG onto the adsorbent could be described by the Freundlich model. It was found that the n value in Freundlich equation is ˂1, indicatingthat the process is cooperative because the value of the slope 1/n is above unity.
Table 1: Isotherm model constants for the adsorption of MG onto DPF
|
Temperature |
Isotherm |
Constants |
R2 |
||
| 25°C (298K) | Langmuir |
Qₒ (mol g-1) |
b (L mol-1) |
0.9547 |
|
|
-3.3336 |
-0.18692 |
||||
| Freundlich |
Kf (L/g) |
n |
1/n |
0.9507 |
|
|
6.74 |
0.73 |
1.369 |
|||
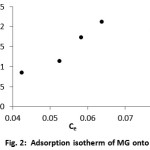 |
Figure 2: Adsorption isotherm of MG onto DPF |
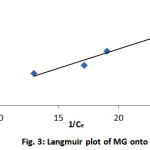 |
Figure 3: Langmuir plot of MG onto DPF |
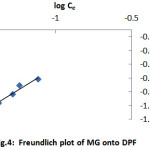 |
Figure 4: Freundlich plot of MG onto DPF |
Effect of pH
Fig. 5 illustrates the effect of pH on the removal of MG dye by DPF in the pH range 4-10.It is noticeable from the figure that the maximum removal is 95.725% at pH = 10 and the removal of dye increases with increasing pH. This result could be explained by the attraction forces between different charges; the adsorbent surface becomes negatively charged in alkaline media, resulting in an attraction between the positively charged dye molecule and the negatively charged adsorbent. In acidic media, the presence of excess H+ ions competing with the positive charge of dye for the adsorption sites, and therefore the removal of dye slows down.
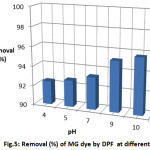 |
Figure 5: Removal (%) of MG dye byDPF at different pH |
Effect of Temperature
Fig. 6 gives information about the percentage removal of the MG by DPF at different temperatures. As observed, the percentage removal decreases slightly with increase in temperature from 25 to 45 °C and the maximum removal is 94.3% at 25ᵒC. A similar trend was reported by Chandra et al. [17] for the adsorption of MB onto activated carbon prepared from durian shell; the researchers suggest that the physical bonding between the organic compound (including the dye) and the active sites on the adsorbent weakens as the temperature increases. In addition, the dye solubility also increases, which causes the interaction between the solute and the solvent to become stronger than that between the solute and adsorbent. Therefore, the solute is less likely to be adsorbed. The highest MG removal of 94.3% was achieved at 25 °C
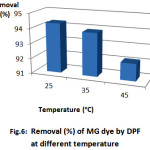 |
Figure 6: Removal (%) of MG dye byDPF at different temperature |
Kinetic Study
Two models were used to analyze the adsorption kinetics of MG by DPF at 25 °C. The first model was pseudo-first-order, and therefore the Lagergren equation was applied. It is given as follows [18]:
![]()
whereqe is the adsorption capacity at equilibrium, qt is the adsorption capacity at time t and k1 is the rate constant. A plot of ln(qe−qt) versus t is linear and K1 could be calculated from the slope while qe could be determined from the intercept of the plot (the figure is not shown). The adsorption process could not be described using the model becausethe valueof correlation coefficient R2was low and the experimental qevalue did notagreewith the value calculatedusingthe equation.
Second model was based on the linear pseudo-second-order equation. It is given as follows [19]:
![]()
where k2 is the pseudo-second-order rate constant. The slope of the plot of t/qt versus t gives the value of qe, and the intercept can be used to calculate K2. The plot of t/qt versus t yields a straight line, as shown in Fig. 7. The calculated value of qe for the pseudo-second-order model is 0.080 (mol g-1), which agrees well with the experimental value at 25ᵒC (= 0.0858mol g-1). Moreover, theR2 value for this model is 0.999, indicating an excellent fit. The value of K2 is found to be 454.215(gmol-1min-1).
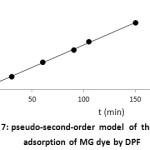 |
Figure 7: pseudo-second-order modelof the kinetic adsorption of MG dye by DPF |
Conclusion
In this work, we studied the adsorption of MG from aqueous solution over a range of MG concentrations on DPF surface. The equilibrium adsorption data was analyzed according to Langmuir and Freundlich isotherms. The effect of temperature and pH were studied by calculating the percentage removal of MG. Better color removal occurred at low temperatures and inbasic media. The kinetic studies revealed that the pseudo-second-order was a better fit than thepseudo first-order model.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Princess Nourahbint Abdulrahman University for funding this work.
References
- Ahmad, M.A., Alrozi, R. , Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies Chemical Engineering Journal, 2011, 171 ,510– 516.
CrossRef - Berberidou, C., Poulios, I., Xekoukoulotakis, N.P., Mantzavinos, D., Sonolytic, photocatalytic and sonophotocatalytic degradation of malachite green in aqueous solutions, Appl. Catal. B ,2007,74 ,63–72.
CrossRef - Allen S.J., Koumanova B., Decolourisation of water/ wastewater using adsorption (review), Journal of the University of chemical technology and metallurgy, 2005, 40, 3, 175-192.
- Hameed, B.H., & El-Khaiary M.I., Removal of basic dye from aqueous medium using a novel agricultural waste material: Pumpkin seed hull. Journal of Hazardous Materials, 2008, 155,601–609.
CrossRef - Dávila-Jiménez , M.M, Elizalde-González, M.P., & Hernández-Montoya, V. , Performance of mango seed adsorbents in the adsorption of anthraquinone and azo acid dyes in single and binary aqueous solutions.Bioresource Technology, 2009, 100, 6199–6206.
CrossRef - Tunç, Ö., Tanacı, H., & Aksu, Z., Potential use of cotton plant wastes for the removal of Remazol Black B reactive dye. Journal of Hazardous Materials, 2009, 163, 187–198.
CrossRef - Hameed, B.H., Mahmoud, D.K., & Ahmad, A.L., Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch wasteJournal of Hazardous Materials, 2008, 158, 65–72.
- Khaled, A., El Nemr, A., El-Sikaily, A., &Abdelwahab, O. , Treatment of artificial textile dye effluent containing Direct Yellow 12 by orange peel carbon,Desalination , 2009, 238, 210–232.
CrossRef - Hameed, B.H., Krishni, R.R., & Sata, S.A., A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. Journal of Hazardous Materials , 2009, 162, 305–311.
CrossRef - Al-Oqla FM, Sapuan SM, Natural fi ber reinforced polymer composites in industrial applications: feasibility of date palm fi bers for sustainable automotive industry. J Clean Prod, 2014, 66:347–354.
CrossRef - Tan, A.W., Ahmad, A.L., & Hameed, B.H., Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. Journal of Hazardous Materials , 2008, 154, 337-346.
CrossRef - Salleh M.A.M., Mahmoud D. Kh., Abdul Karim W.A. W.,Idris A., Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review, Desalination,2011, 280, 1–13.
CrossRef - Giles, C.H., Macewan, T.H., & Smith, D.J., Studies in Adsorption. Part XI. A System of Classification ofSolution Adsorption Isotherms, and its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids.Journal of the Chemical Society. 1960, 3973-3993.
CrossRef - El-Guendi, M., Homogeneous surface diffusion model of basic dyestuffs onto natural clay in batch adsorbers. Adsorption Science and Technology, 1991, 8, 2, 217-225.
CrossRef - Desta, M.B., Batch sorption experiments: Langmuir and Freundlich isotherm studies for adsorption of textile metal ions TeffStraw(Eragrostistef) agricultural waste, Journal of Thermodynamics,2013,Article ID 375830,1-6.
- El-Sayed, G.O., Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber, Desalination ,2011, 272, 225–232.
CrossRef - Chandra, T.C., Mirna, M.M., Sudaryanto, Y.,& Ismadji, S., Adsorption of basic dye onto activated carbon prepared from durian shell: Studies of adsorption equilibrium and kineticsChemical Engineering Journal , 2007, 127, 121-129.
- Lagergren, S., About the theory of so called adsorption of soluble substances, Ksver Veterskapsakad Handl, 1898,24, 1-6.
- Ho, Y.S., McKay, G., Sorption of dye from aqueous solution by peat.Chemical Engineering Journal , 1998, 70, 115-124.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









