The Development of Centellaasiatica Extract-Loaded BSA Nanoparticles Production to Improve Bioavailability
Kittiya Kesornbuakao1 and Patchanee Yasurin2*
1Food Technology Department, Biotechnology Faculty, Assumption University, Bangkok, 10240, Thailand.
2Food Biotechnology Program, Biotechnology Faculty, Assumption University, Bangkok, 10240, Thailand.
Corresponding Author E-mail: patchaneeYsr@au.edu
DOI : http://dx.doi.org/10.13005/ojc/320513
Centellaasiatica (Bao-bog, Pennywort, Gotu kola) is used as a traditional drug widely in Asia. C. asiatica crude extracts showed excellent potential in-vitro but less or no in-vivo activity due to their poor lipid solubility or improper molecular size or both, resulting in poor absorption, poor dosing and poor bioavailability. The Bovine serum albumin (BSA) can attract macromolecular and carry wide variety of molecule. So this research was aimed to develop C. asiatica extract-loaded BSA nanoparticles (CBNP) to improve bioavailability. CBNP was prepared by the desolvation method using three different ratio C. asiatica crude chloroform extracts: BSA (1:2, 1:3, and 1:4). The well agar diffusion method was used for evaluating antibacterial activity of CBNP with different concentration (100, 200, and 300 µg/ml) against five food borne pathogens (Escherichia coli ATCC25822, Salmonella entericaTyphimurium U302 (DT104b), S. entericaEnteritidis (human), S. enterica 4,5,12:i:- (human) US clone, and Bacillus cereus). The results showed that the antibacterial activity of CBNP did not show significant different onthree different ratio and concentration in all food borne pathogens except S. entericaEnteritidis (human) and B. cereus (P < 0.05). The highest antibacterial active of CBNP was 1.07±0.46 cm against S. entericaEnteritidis (human) on ratio 1:4, 200 µg/ml. The antibacterial activity of CBNP gave almost 2 times higher than free crude C. asiatica chloroform extracts. The modified Folin–Ciocalteu method, Ferric reducing antioxidant potential assay and DPPH assay were used for evaluating antioxidant activity. The highest antioxidant activity represented by the amount of phenolic content of CBNP was 14.59±6.74 µgGAE/mg using ratio 1:2.The amount of phenolic content of CBNP did not show significant different between ratio 1:2 and others (P < 0.05). For Ferric reducing antioxidant potential assay and DPPH assay, crude chloroform extract showed significantly higher activity than CBNP were 1.00 ± 0.15 mmol Fe2+/mg and 29.44 ± 8.20 % DPPH radical scavenging respectively (P < 0.05). The entrapment efficiency and loading efficiency of CBNP showed highest value on ratio 1:4 which were 96.94 ± 1.48% and 42.05 ± 5.68% respectively. It’s also showed highest in release kinetic in Vitro approximately 12% during the whole period of 6 hours in both artificial gastric and intestinal juice. C. asiaticacrude chloroform extract have higher solubility in water than CBNP but CBNP have higher stability in releasing crude chloroform extract. The results indicated that CBNP showed the promising to increase bioavailability of C. asiatica. The statistical analysis was done by ANOVA using SAS software version 9.3.
KEYWORDS:Centellaasiatica; Nanoparticles; Bioavailability; Antibacterial; Antioxidant
Download this article as:| Copy the following to cite this article: Kesornbuakao K, Yasurin P. The Development of Centellaasiatica Extract-Loaded BSA Nanoparticles Production to Improve Bioavailability. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Kesornbuakao K, Yasurin P. The Development of Centellaasiatica Extract-Loaded BSA Nanoparticles Production to Improve Bioavailability. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21858 |
Introduction
C. asiatica(Bao-bog) is herbal in Asia. In Ayurvedic, an Indian system of medicine, C. asiaticais used for the treatment of leprosy, insanity, asthma, ulcers, eczema, skin and gastrointestinal disorder, arthritis, varicose vein and high blood pressure1. The major biologically active compounds of C. asiaticaextract are monoterpenes, sesquiterpene, and triterpenoids2. It was found that the chemical complexity of the herbal extracts seem to be important for the bioavailability. Even though, C. asiaticanano powder gave higher yield of active compound (Asiatic acid) than natural C. asiatica powder3there have been some limitations in C. asiatica crude extracts that it showed their extra ordinary potential in-vitro but less or no in-vivo activity due to their poor lipid solubility and improper molecular size, resulting in poor absorption, slow delivery, poor dosing and poor bioavailability. Especially, it is also difficult for water-soluble biological active compounds to enter through cell membrane of both human and pathogenic microorganism, which has hydrophobic characteristics. Albumin is a protein that can be obtained from a variety of sources, including egg white (ovalbumin), bovine serum albumin (BSA), and human serum albumin (HSA). Albumin is the major soluble protein of the circulating system and involved in the maintenance of osmotic pressure and binding and transport of nutrients to the cells. Albumin can soluble in water and diluted salt solution very well4. The high solubility of albumin (up to 40% w/v). Albumin is stable in the pH range of 4 to 9 and can be heated at 60°C up to 10 hours without any deleterious effects and at pH 7.4 makes it an attractive macromolecular carrier capable of accommodating a wide variety of molecule5. The development of CBNP are the guild line to overcome these problems. Not only improve drug delivery system but the CBNP also reduce cost from purification steps, and can be applied to use in high valued industry products. Therefore, the objective of this research is to develop CBNP on antibacterial activity, antioxidant activity, and nanoparticles properties.
Materials Methods
Preparation of Sample
C. asiaticawas purchased from local markets in Bangkok, Thailand. The aerial part of C. asiaticawas used. Fresh C. asiatica were washed with tap water and cut into small pieces. Then it was air dried in oven (Memmert UM500) at 45°C. The dried samples was finely ground into powder. The powder were kept at 4°C before used.2
Preparation of C. Asiatica Crude Chloroform Extract
C. asiaticawas extracted with chloroform using 1:10 ratio (g/ml). The mixture was macerated at room temperature, 120 rpm, for 48 hours and then it was filtered using whatman filter paper no.4. The crude extract was concentrated using rotary evaporators at 45°C (BUCHI Rotavapor R-205) are then it was kept at -20°C before use.2 The C. asiaticacrude chloroform extracts was further used for preparation of CBNP.
Preparation of C. Asiatica Extract-Loaded BSA Nanoparticles
CBNP was prepared by the desolvation method6. The 100 mg of BSA were dissolved in 1 ml of sodium chloride solution (10mM). Then, 8.0 ml of ethanol was added dropwise into the BSA solution under magnetic stirring (400 rpm) at room temperature. Subsequently, the as-prepared BSA nanoparticles were cross-linked with 0.2% glutaraldehyde (GA). Then, C. asiaticacrude chloroform extract was added into the solution for 24 hours at different ratio of C. asiatica to BSA (1:2, 1:3, and 1:4) in the preparation of CBNP. The particles were centrifuged and washed with distilled water. The centrifuged particles were resuspended and disperse in 2% mannitol, then freeze-dried for 24 hours. The dried nanopowder werekept at room temperature before use.
Antimicrobial Activity
The modified agar well diffusion method2 is used. The 100 μl of bacteria (approximately 1.5 × 108 CFU/ml) is swab on Mueller-Hinton agar (MHA) plate. The 50μl ofC. asiaticacrude chloroform extract and CBNP at concentration 100, 200, and 300 µg/ml diluted with distilled water were used to test antibacterial activity against Escherichia coli ATCC25822, Salmonella entericaTyphimurium U302 (DT104b), S. entericaEnteritidis (human), S. enterica 4,5,12:i:- (human) US clone, and Bacillus cereus. The 20μl of 50 mg/ml penicillin G was used as positive control. The inhibition zones were measured to determine the effectiveness of the C. asiaticacrude extract and CBNP against each bacterium. The experiment was done in duplicate and three replication independently.
Antioxidant activity by Total Phenolic Content
The modified Folin–Ciocalteu method7was used for total phenolic content determination of C. asiaticacrude chloroform extract and CBNP. The 20 μl of 10 mg/ml C. asiaticacrude chloroform extract and CBNP was added to 1.58 ml distilled water and 100 μlFolin–Ciocalteu phenol reagent. The mixture was then allow to stand for 8 minutes 30 seconds and 300 μl saturated sodium carbonate solution was added to the mixture. Then the mixture was incubated without light at room temperature for 30 minutes and observed optical density (OD) at 765 nm. The result were expressed as microgram garlic acid equivalent (µgGAE/ml). The experiment was done in triplicate and three replicationindependently.
Antioxidant Activity by Ferric Reducing Antioxidant Potential Assay (FRAP)
The modified ferric reducing antioxidantpotential assay8 was used to determine FRAPvalue of C. asiatica crudechloroform extract and CBNP. The FRAPreagent was prepared using 300 mmol sodiumacetate buffer at pH 3.6, 20 mmol iron chloride and10 mmol 2,4,6-tripyridyl-s-triazine dissolved in 40mmol hydrochloric acid at a ratio of 10:1:1 (v:v:v).The reagent was incubated at 37°C for 10 minutes before use. The 20μl of 1 mg/ml the extract and CBNP wasadded, followed by adding 1000μl of FRAP reagentvigorously and kept in the dark for 30 minutes. The optical density (OD)of this mixture was measured at 593nm. FRAP values were expressed as mmol Fe2+/mg of sample. All measurements were done intriplicate and three replications independently.
Antioxidant Activity by DPPH Radical Scavenging Activity
The modified DPPH radical scavenging activity9was used for percentage DPPH radical scavenging determination.The 100 μl of 1 mg/ml C. asiatica crude chloroform extract and CBNP were mixed with 3.9 ml DPPH reagent (50 μM). The mixture was shaken vigorously and allowed to stand at room temperature in the dark for 30 minutes. The optical density (OD) was measured at 517 nm. The results were expressed as percentage reduction of DPPH10.
![]()
Where A0 is the initial absorbance and Ac is the value for added sample concentration c.All measurements were done intriplicate and three replications independently.
Entrapment Efficiency and loading Efficiency
C. asiatica crude chloroform extract was run absorbance spectrum to find the best the wavelength (λmax) at which the absorbance is the greatestby UV-vis spectrophotometer. The 2 mg CBNP were dissolved in 1 ml methanol and gently shaken for 24 hours at 37 °C to completely extract C. asiatica crudechloroform extract to methanol11.Then the solutions were centrifuged at 12000 rpm for 10 min, and the supernatant was kept and measured optical density (OD) by a UV-vis spectrophotometer at λmax. The amount of C. asiatica crudechloroform extract entrapped and loaded in CBNP is express as entrapment efficiency and loading efficiency calculated as follows11:

All measurements were done intriplicate and three replications independently.
Solubility and Stability
To compare the solubility of C. asiatica crude chloroform extract before and following the encapsulation process, saturation solubility is determined11. Excessive samples (C. asiatica crudechloroform extract and CBNP) were dispersed into 1 mL water and at 200 rpm, 37 °C. After 24 hours, samples were taken out and filtered through a 0.22 μm Millipore membrane. Filtrate was diluted appropriately, and the optical density (OD) were measured by a UV-vis spectrophotometer at λmax.
The 1 mg/mL of CBNP in phosphate buffer solution (0.01M, pH7.4) were incubated at 200 rpm, 37 °C, for 24 hours. At designated time points (0, 0.5, 1, 2, 3, 4, 5, 6 hours), the mixture was sampled and the optical density (OD) was measured by UV-visspectrophotometer at λmax11. The stability of CBNP is calculated as follows:
![]()
Where C0 is the initial absorbance and Ct is theabsorbance of the sample at time point.All measurements were done intriplicate and three replications independently.
Release kinetic in Vitro
Release kinetic11 methodology was modified. The release of C. asiatica crude chloroform extract from CBNP was done by dissolving 20 mg of CBNP in 15 ml artificial gastric juice (0.01 M PBS pH 2.0) and intestinal juice without enzymes (0.01 M PBS pH 7.4). The mixture is incubated at 37 °C at 200 rpm. At designated time points (0, 0.5, 1, 2, 3, 4, 5, 6 hours),mixture is sampled and centrifuged at 3000 rpm for 10 min. The pellet is resuspended in 100 μL of methanol to determine the amount of C. asiatica crude chloroform extract released by measuring optical density (OD) by UV-visspectrophotometer at λmax..All measurements were done intriplicate and three replications independently.
Statistical Analysis and Experimental Design
All experiments were conducted in three replications and statistical analysis wasaccomplished using ANOVA with Duncan’s multiple range tests (p < 0.05) by SAS software version 9.3.
Results and Discussion
Antimicrobial Activity
CBNP were prepare by a desolvationmethod6. Nanoparticles are taken up by cells more efficiently than larger micromolecules and therefore, could be used as effective transport and delivery systems. The strategy of applying nanotechnology to plant extracts has been widely used, because nanostructure systems could increase effect of action of plant extracts, promote sustained release of active constituents, reduce the required dose, decrease side effects, and improve activity12, 13.There are three different of the nanoparticles preparative. The different between these three nanoparticles were ratio of C. asiaticacrude chloroform extracts to BSA 1:2, 1:3, and 1:4. The well agar diffusion method2 was used for evaluating antibacterial activity of CBNP and C. asiatica crude chloroform extracts with different concentration (100, 200, and 300 µg/ml) against five food borne pathogens (Escherichia coli ATCC25822, Salmonella entericaTyphimurium U302 (DT104b), S. entericaEnteritidis (human), S. enterica 4,5,12:i:- (human) US clone, and Bacillus cereus).
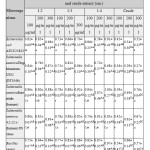 |
Table 1: Antibacterial activity as inhibition zone (cm) of CBNP and crude extract against different microorganisms |
Note: Different capital letter (A, B, C) superscript within a row showed significant different at p<0.05.Different small letter (a, b, c) superscript within a column showed significant different at p<0.05.
The results of CBNP and C. asiaticacrude chloroform extracts antibacterial activity were interpreted by using Randomized Complete Block Design (RCBD) with Duncan’s multiple range tests in SAS program version9.3 as showed in table1. It was found that antibacterial activities trend of CBNP increased from C. asiaticacrude chloroform extracts about 2-3 times significantly(p<0.05). C. asiatica crude chloroform extracts have low antibacterial activity because there is a hydrophilic active compound so, it is difficult for water-soluble biological active compounds to permeate through cell membrane, which has hydrophobic characteristics. But, bovine serum albumin (BSA) of CBNP is the protein can attract macromolecular and carry variety of molecule of active compound5.Albumin nanoparticles are formed by linkage of amino acid by electrostatic and attraction and covalent linkage reagent (e.g. glutaraldehyde, genipin) and can be prepare to a size of between 100-200 nm14. It also can readily bind and release small molecule5. All of these reasons made it can increase an efficiency of absorption to the cells. Moreover, CBNP tend to have more significantly effect on gram-negative bacteria (E. coli ATCC25822, S.entericaTyphimurium U302 (DT104b), S. entericaEnteritidis (human), and S. enterica 4, 5, 12: i: – (human) US clone)than gram-positive bacteria (B. cereus). Gram-positive and gram-negative bacteria have difference structural in cell wall composition15. In Gram positive bacteria, there is an inner membrane which is surrounded by a thick cell wall made of peptidoglycan16. In Gram negative bacteria, there is an inner membrane followed by a thin peptidoglycan layer and an outer membrane16. Furthermore, it was found that the different ratio of C. asiatica crude chloroform extracts: BSA and concentration showed no significant difference in antibacterial activity of CBNP against all pathogens except S. entericaEnteritidis (human) and B. cereus (P < 0.05).The highest antibacterial active was 1.07±0.46 cm using CBNP ration 1:4, 200 µg/ml against S. entericaEnteritidis (human). The encapsulation process of CBNP can improve antibacterial activity of C. asiatica crude chloroform extractsagainst the pathogens.
Antioxidant Activity
The antioxidant activity can be measured by the ability of the compound that can catch free radicals compounds by scavenging or trapping methods17. Herb and spice are rich in phenolic compounds18 which are an active compound that have antioxidant properties as protective agent against free radical compound19.The majorbiologically active compounds of C. asiatica extract are monoterpenes, sesquiterpene,triterpenoids2, polyphenols20 and triterpenes21. In addition toterpenoids, it also contains high total phenolic contents which contributed by the flavonoidssuch as quercetin, kaempherol, catechin, rutin, apigenin and naringin and volatile oils such ascaryophyllene, farnesol and elemene20, 22.The antioxidant activity of C.asiatica may be due to the reduction of hydroperoxides, inactivation of free radicals, chelation of metal ions or combinations20. The antioxidants present in theC. asiatica may have different functional properties, such as reactive oxygen speciesscavenging (quercetin and catechins) 23, inhibition of the generation of free radicals and chain-breaking activity, e.g. p-coumaric acids24 and metalchelation25.
Table 2: Total phenolic content , Ferric reducing antioxidant potential, and DPPH radical scavenging of CBNP and C. asiaticacrude chloroform extract
|
CBNP with different ratio between Crude:BSA and crude |
Total phenolic content (µg GAE/mg dried weight) |
FRAP (mmol Fe2+/mg dried weight) |
DPPH (% reduction of DPPH) |
|
|
1:2 |
14.59 ± 6.74A |
0.53 ± 0.16B |
11.55 ± 4.70B |
|
|
1:3 |
13.15 ± 5.94A |
0.49 ± 0.06B |
13.57 ± 8.99B |
|
|
1:4 |
13.15 ± 7.13A |
0.50 ± 0.01B |
5.76 ± 10.44B |
|
|
crude |
13.15 ± 1.62A |
1.00 ± 0.15A |
29.44 ± 8.20A |
|
Note: Different superscript within a column showed significant different at p<0.05
The modified Folin–Ciocalteu method7, Ferric reducing antioxidant potential assay8 and DPPH radical scavenging9 were used for evaluating antioxidant activity of CBNP and C. asiatica crude chloroform extracts. The results were interpreted by using Randomized Complete Block Design (RCBD) withDuncan’s multiple range tests in SAS program version9.3as showed in table2.The phenolic compound was bind to BSA mainly by hydrogen bond, electrostatic, and hydrophobic interaction. Molecular structure and the number of hydroxyl group of phenolic compound are also the factor that effect phenolic compound binding with BSA26. Total phenolic content of CBNP and crude extract were determined in comparison with standard garlic acid and the results were expressed in terms of µg GAE/mg dried weight. The highest antioxidant activity represented by the amount of phenolic content of CBNP was 14.59±6.74 µgGAE/mg using ratio 1:2.The amount of phenolic content of CBNP did not show significant different between ratio 1:2 and others (P < 0.05). For Ferric reducing antioxidant potential assay and DPPH assay, crude chloroform extract showed significantly higher activity than CBNP were 1.00 ± 0.15 mmol Fe2+/mg and 29.44 ± 8.20 % DPPH radical scavenging respectively (P < 0.05).After process of nanoparticles, C.asiatica loss of its activity in ferric reducing antioxidant potential assay and DPPH radical scavenging. Because of the active compound maybe are already bind with protein and changed the structural to be inactive form.
Table 3: Entrapment efficientcy, loading efficiency, and solubility of CBNP and C. asiaticacrude chloroform extract
|
CBNP with different ratio between Crude:BSA and crude |
Entrapment efficiency (%) |
Loading Efficiency (%) |
Solubility (µg/ml) |
|
|
1:2 |
42.77 ± 4.23B |
41.28± 8.91A |
203.58 ± 113.81A |
|
|
1:3 |
59.48 ± 8.90B |
36.60 ± 7.56A |
172.59 ± 13.53A |
|
|
1:4 |
96.94 ± 1.48A |
42.05 ± 5.68A |
168.86 ± 78.78A |
|
|
crude |
244.34 ± 17.10A |
|||
Note: Different superscript within a column showed significant different at p<0.05
Entrapment Efficiency and loading Efficiency
The entrapment efficiency and loading efficiency were calculated using equations 2 and 3 mentioned above. The results were interpreted by using Randomized Complete Block Design (RCBD) withDuncan’s multiple range tests in SAS program version9.3as showed in table3. CBNP using ratio 1:4 has entrapment efficiency significantly higher than ratio 1:3 and 1:2 which were 96.94 ± 1.48%, 59.48 ± 8.90%, and 42.77 ± 4.23% respectively (p<0.05). There are no significantly difference in entrapment efficiency between CBNP using ratio 1:3 and 1:2 (p<0.05). For loading efficiency, there are no significantly different among ratio of CBNP (p<0.05). CBNP using ratio 1:4 also has highest percentage followed by 1:2 and 1:3 which were 42.05 ± 5.68%, 41.28± 8.91%, and 36.60 ± 7.56% respectively.
Solubility and Stability
To determine the solubility of C. asiaticacrude chloroform extracts before and following the encapsulation process, C. asiaticacrude chloroform extracts and CBNP were dissolved in water. The results were interpreted by using Randomized Complete Block Design (RCBD) withDuncan’s multiple range tests in SAS program version9.3as showed in table3. The result showed that C. asiaticacrude chloroform extracts has highest solubility in water which is 244.34 ± 17.10µg/ml. But, there are no significant difference between C. asiaticacrude chloroform extracts with others CBNP (p<0.05). While, CBNP using ratio 1:4 has lowest solubility which is 168.86 ± 78.78 µg/ml. The process of making CBNP improve hydrophobic capacity of crude extract. It is difficult for water-soluble biological active compounds to enter through cell membrane of both human and pathogenic microorganism, which has hydrophobic characteristics. So, improving of hydrophobic capacity of crude extract make it can be better in entering through the cell.
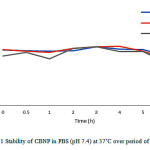 |
Figure 1: Stability of CBNP in PBS (pH 7.4) at 37˚Cover period of 6 hours. |
To study the stability of CBNP, it was incubated in PBS (0.01M, pH 7.4) and calculate their concentration with time by equation 4. CBNP showed very stable under the condition over period of 6 hours as showed in figures1. The nanoparticles process made the stability of C. asiaticacrude chloroform extracts by protecting it from hydrolysis and biotransformation11. CBNP provided stability of the C. asiatica crude extract so, it can be used as process to prolong shelf-life of plant extract.
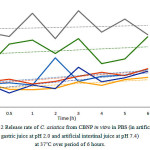 |
Figure 2: Release rate of C. asiatica from CBNP in vitro in PBS (in artificial gastric juice at pH 2.0 and artificial intestinal juice at pH 7.4)at 37˚C over period of 6 hours.
|
Release Kinetic in vitro
In the study of release kinetic in vitro, artificial gastric juice (0.01 M PBS pH 2.0) and artificial intestinal juice (0.01 M PBS pH 7.4) were used to imitate the environment in stomach and intestine. The cumulative percentage of released C. asiaticawere determine as showed in figure2.The release rate of C. asiatica from CBNP using same ratio in the gastric juice was tend to higher than intestinal juice. CBNP using ratio 1:4 released highest in both artificial gastric juice (0.01 M PBS pH 2.0) and artificial intestinal juice (0.01 M PBS pH 7.4). At pH 2.0 the BSA protein was unfolded and release C. asiatica out from the particles. BSA was most stable at pH 7 but rapidly degraded by aggregation and hydrolysis at pH 227.CBNP can be release in very low pH so, it can be apply to be oral medicine28.
Conclusions
The different ratio of C. asiatica to BSA (1:2, 1:3, and 1:4) has effect on bioavailability.CBNP were less solubility in water than crude extractthus, CBNP can improve hydrophobic capacity of C. asaiticacrude extract.So, this study is focus on improving ability of C. asaiticacrude extract in absorption to the cell.Higher hydrophobic capacity of the CBNP makes it can penetrate into cell membrane and enter to the cell.These result is parallel to antibacterial activity of CBNP that showed 2 times higher than crude extract. Moreover, CBNP has no significantly different in total phenolic content compare with crude extract, but has significantly lower in FRAP and DPPH (p<0.05). CBNP also showed stability and releasing of C. asiatica in PBS.It can be conclude that CBNP at ratio 1:4 is most effective in an economical way because using less crude extract. The development of CBNP would be a promising improving bioavailability of C. asaiticacrude extract.
Acknowledgements
This research is supported by the Assumption University (research grant P-58-388).
References
- Ariffin, F.;Heong, Chew S.; Bhupinder, K.; Karim, AA.; Huda, N. Antioxidant capacity and phenolic composition of fermented Centellaasiatica herbal teas. J Sci Food Agric. 2011, 91, 2731-9
- Rattanakom, S.;Yasurin, P. Antibacterial activity, antioxidant activity and chemical profiling of Centellaasiatica under different extraction solvents. Orient. J. Chem. 2015, 31, 2453-2459
- Borhan, M. Z.; Nee, T. Y. Synthesis of TiO2nanopowders from red gypsum using EDTA as complexing agent. Journal of Nanostructure in Chemistry. 2014
- Lohcharoenkal,Warangkana.;Wang,Liying.; Chen, Yi Charlie.;Rojanasakul, Yon. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy.BioMed Research International. 2014
- Kratz, F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. PubMed. 2008 ,132, 171-83
- Yu, Z.; Yu, M.; Zhang, Z.; Hong, G.;Xiong, Q. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Research Letters. 2014, 9, 343
- Ragazzi, E.; Veronese, G. Quantitative analysis of phenolic compound after thin-layer chromatographic separation. J Chromatogr A.1973, 77, 369-75
- Benzie, IF.; Strain, JJ. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15-27
- Brand-Williams, W.;Cuvelier, ME.;Berset, C.Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology. 1995, 28, 25-30
- Molyneux, P.The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211-219
- Xie, X.; Tao, Q.;Zou, Y.; Zhang, F.;Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu,Shuqin. PLGA Nanoparticles Improve the Oral Bioavailability of Curcumin in Rats: Characterizations and Mechanisms. Journal of Agricultural and Food Chemistry. 2011, 59, 9280–9289
- V, Ghosh.;S,Saranya.;A,Mukherjee.; N,Chandrasekaran. Antibacterial microemulsion prevents sepsis and triggers healing of wound in wistar rats. Colloids and Surfaces B: Biointerfaces. 2013, 105, 152–157
- Rajendran, R.;Radhai, R.;Kotresh, TM.;Csiszar, E. Development of antimicrobial cotton fabrics using herb loaded nanoparticles. Carbohydrate Polymers. 2013, 91, 613-617
- Weber, C. Desolvation process and surface characterisation of protein nanoparticles. International Journal of Pharmaceutics. 2000, 194, 91-102
- Madigan, M.;Martinko, J. Brock biology of microorganisms. 11th ed. Englewood Cliffs. NJ: Prentice Hall. 2005
- Brown,Lisa.; M,Julie.; Wolf, Rafael Prados-Rosales. Arturo Casadevall Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi Nature Reviews Microbiology.2015, 13, 620–630
- Huang, D.;Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem.2005, 53, 1841-1856
- NV, Yanishlieva.; E,Marinova.; J,Pokorny. Natural antioxidant from herb and spices. Eur J Lipid Sci Technol.2006, 108, 776-93
- D, de Beer.; E, Joubert.; W.C.A., Gelderblom.; M, Manley. Phenolic Compounds: A Review of Their Possible Role as In Vivo Antioxidants of Wine.2002, 23
- MK,Zainol.; A,Abd-Hamid.; S,Yusof.; R,Muse. Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centellaasiatica(L.) Urban. Food Chemistry.2003, 81, 575-581
- Inamdar, P.K.; Yeole, R. D.; Ghogare, A. B.; de Souza, N. J.Determination of Biologically Active Constituents in Cente-llaAsiatica. J. Chromatogr. A.1996, 742, 127-30
- Chong, NJ.; Aziz, Z.A Systematic Review of the Efficacy of Centellaasiatica for Improvement of the Signs and Symptoms of Chronic Venous Insufficiency. EvidencebasedComplementary and Alternative Medicine: eCAM. 2013, 627182.10.1155/627182
- Hatano,T.;Edamatsu, R.;Hiramatsu, M.;Moti, A.; Fujita, Y.;Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-diphenyl-2-picrylhydrazyl radical. Chemical and Pharmaceutical Bulletin.1989, 37, 2016-2021
- Laranjinha, J.; Vieira, O.; Madeira, V.; Almeida, L. Two related phenolic antioxidants with opposite effects on vitamin E content in low density lipoproteins oxidized by ferrylmyoglobin: consumption vs regeneration. Archives of biochemistry andbiophysics.1995, 323, 373-381
- Van Acker, SA.; Van Balen, GP.; Van den Berg, DJ.;Bast, A.; Van der Vijgh, WJ. Influence of iron chelation on the antioxidant activity of flavonoids.Biochemicalpharmacology.1998, 56, 935-943
- Tianxi, He.;Qionglin, Liang.;Tingting, Luo.;Yiming, Wang.;Guoan,Luo. Study on Interactions of Phenolic Acid-Like Drug Candidates with Bovine Serum Albumin by Capillary Electrophoresis and Fluorescence Spectroscopy. Journal of Solution Chemistry.2010, 39, 1653-1664.
- Tia, Estey.;Jichao, Kang.; Steven P., Schwendeman.; John F., Carpenter. BSA Degradation under Acidic Conditions:A Model for Protein Instability during Release from PLGA Delivery Systems. Journal of pharmaceutical sciences.2006, 95.
- Yasurin, P.; Sriariyanun, M.; Phusantisampan, T. Review: the bioavailability activity of Centella asiatica. KMUTNB Int J Appl Sci Technol. 2016, 9, 1-9.

This work is licensed under a Creative Commons Attribution 4.0 International License.









