Synthetic Studies and Antibacterial Activity of Nucleobases and their N- and S-Glucosidesfrom 2-Amino Benzoic Acid and its Benzamido Derivatives
Samia Benhammadi, Salimairaten and Adil A. Othman
Faculty of Chemistry, University of Science and Technology of Oran-USTO- B.P. 1505 Oran El M'Naouer. 31003, Oran, Algeria.
Corresponding Author E-mail: adelaliothman@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320528
A series of S-glucosides11a-14a and their benzamido derivatives 11b-14b have been synthesized by reacting D-glucose withthiol groups of 5-(2’-aminophenylene)-1,3,4-oxadiazole-2-thioles7(a,b), 5-(2’-aminophenylene)-1,3,4-thiadiazole-2-thiols8(a,b), 5-(2’-aminophenylene)-1,2,4-triazole-3-thiols9(a,b)and 5-(2’-aminophenylene)-4-N-amino-1,2,4-triazole-3-thiols10(a,b). The thiols7(a,b)-10(a,b) have been synthesized from hydrazides3(a,b)which already been synthesized from 2-aminobenzoic acid and its benzamido derivative.Allsynthesized compoundswerecharacterized by IR, UV,1H- and13C- NMR. Nucleobases and a representative of S-glycoside weretestedin vitro against the following microorganisms: twoGram-positive bacteria Staphylococcus aureusandBacillus cereusand two Gram-negative bacteria Escherichia coli, Pseudomonasaeruginosaand they exhibited significant effects.Amykacinewas used as positive standard.
KEYWORDS:Nucleobases; glycosides; benzamide derivatives; synthesis; antibacterial activity
Download this article as:| Copy the following to cite this article: Benhammadi S, Salimairaten S, Othman A. A. Synthetic Studies and Antibacterial Activity of Nucleobases and their N- and S-Glucosidesfrom 2-Amino Benzoic Acid and its Benzamido Derivatives. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Benhammadi S, Salimairaten S, Othman A. A. Synthetic Studies and Antibacterial Activity of Nucleobases and their N- and S-Glucosidesfrom 2-Amino Benzoic Acid and its Benzamido Derivatives. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=22616 |
Introduction
Natural nucleosides and glycosides are glycosylamines consisting of nucleobases (often referred to as pyrimidine and purine derivatives). They are bound to a ribose or deoxyribose sugar via a beta-glycosidic linkage.1 In molecular biology, several analogues of the sugar backbone exist e.g. locked nucleic acid (LNA) and peptide nucleic acid (PNA)or having different nucleobases like morpholine.2In medicine several nucleosides analogues are used as antiviral,3 anticancer,4 antibacterial5 and antifungal6 agents. Synthetic modified nucleosides designed to pair in unusual ways with natural or unnatural nucleobases have many potential applications in biology; biotechnology and medicine have been reported.7
Since the discovery of nucleoside ribavirin8 whichmade oftriazole as its nucleobase,proved that it is not only possesses inhibitory activity against a range of DNA and RNA viruses 9 but also displays antitumor activity 10 in mice.
The modern synthesis of nucleosides and glycosides covered almost any kind of sugar-beta-glycoside linkage and heterocyclic rings.Recently many C-, O-, N- and S-glycosides11-14have received considerable attention, because they are widely employed as biological inhibitors, inducers, and ligands for affinitychromatography for carbohydrate processing-enzymes and proteins.15
Much attention has been focused onthiol derivatives of1,3,4- oxa, thia-diazoles, 1,2,4-triazole and amino- 1,2,4-triazolefor their broad-spectrum activities, such as antitumor, anticonvulsant, antifungal,16-19 herbicidal, and plant growth regulatory activities.20
Till now, many of these diazole derivatives have been synthesized, and some of them have been patented for commercial uses.21The synthesis and biological evaluation of N-glucosides16,22 and C-glucosidescontaining 1,2,4-triazole have been greatly emphasized,but only a few S-glucosides containing nucleobases of five membered heterocycles with three hetero atoms have been reported.
Recently, it has been reported that 4-benzamidobenzoic acid hydrazide derivatives exhibited considerable in-vitro soluble epoxide hydrolase inhibitory activity.23,24We alsoobserved that the benzamidobenzoic derivatives of all synthesized intermediates had better solubility and showed some different biological effects.
Experimental
General
All reactions monitored by TLC, silicagelF254, made by Merck, Germany.The melting points measured with a BÜCHI 540 melting point apparatus and are uncorrected. The IR spectra exhibited as (wave number: vcm-1) were recorded using KBr discs in a JASCO V-530 spectrophotometer at University of Oran-1, Algeria.The UV spectra were recorded on a ZUZI Split-Beam UV-Vis 4418PC (4418SPC) spectrophotometerexhibitedas (lmax in nm).The 1H NMR and 13C NMR (250 MHz) spectra in DMSO-d6 exhibited as (δ/ppm) were recorded at University of Oran-1, Algeria..
Microorganisms in this study supplied by the university hospital of Oran and identified by the laboratory of applied microbiology, University of Oran-1. The Mueller Hinton medium supplied by (Difco).
Synthesis
General procedures for esterification of 2-amino benzoic acid.( 2-Amino-methyl and 2-amino-ethyl benzoates 2A and2B):
Methyl esterification, catalysed by Argil (Montmorillonite) to give 2-amino methylbenzoate2i
An acid 1 ( 7g., 0.051 mole) dissolved in methanol (200 ml) added to it dried argil – preheated to 100°C- for 1 hr (7.0g) and the mixture was refluxed on water bath with an aid of magnetic stirring for 4 hr. After cooling, a saturated aqueous solution of NaOH (20ml) added and extracted twice with dichloromethane (20 ml). The combined extracts dried over anhydrous Na2SO4, filtered and evaporated to dryness to give amino methyl benzoate2A ascolourless syrup (5.1 g., 65%). Lit m.p. 24°C. IR,nmax/ cm-1 3427 , 1729 (CO).
Ethyl esterification catalysed by H2SO4to give 2-amino ethylbenzoate2B
An acid1 (5g. 0.02 mole) was dissolved in ethanol (200 ml) added to it, drops of H2SO4 (5 ml.). The mixture heated under reflux at 80°C for 8 hours. The reaction was monitored by TLC eluted with chloroform/cyclohexane (6/4) . The reaction mixture was cooled down to room temperature and neutralized by solid NaHCO3, filtered off and washed with alcohol (2 ml.). The filtrate was dried over MgSO4 anhydrous, filtered and washed with alcohol (2ml), the combined filtrates were evaporated to dryness to give 2-amino ethylbenzoate2B as colourless syrup (1.8 g., 30%).Lit m.p. 13°-15°C. IR,nmax/ cm-1 3340 (NH2), 1670 (CO) , UV , lmax 192 nm. Loge 0.117; 194 nm.loge 0.119; 222nm. loge2.067; 248 nm. loge0.763 ; 336 nm. loge0.542
(Benzoamidation of 2- amino benzoic acid (2-Benzoamido benzoic acid (2C
2-Amino benzoic acid 1(5.0 g, 0.033 mole), dissolved in chloroform (100 ml), benzoyl chloride (50 ml) was added gradually, and the mixture was cooled into an ice bath. Pyridine ( 5.0 ml) was added dropwise with an aid of stirring. The reaction mixture was refluxed at 110°C for 10 hr. Reaction was monitored by TLC (CHCl3/cyclohexane, 6:4). Solvents were distilled under vacuum to give crystalline solid which on recrystallization fromethanol/hexane gave a colourless crystalline2-benzamido benzoic acid(2C) (07.92 g, 90 %):M.p. 182-183°C. IR,nmax/ cm-1 3332 (NH), 1697( O-C=O), 1672 (N-C=O).
Esterification of 2-benzoamido benzoic acid (2C),(2-Benzamidomethylbenzoate (2D)
2-Benzoamido benzoic acid(2C) was esterified with methanol and H2SO4–as above- to give white crystalline2- bezamidomethylbenzoate (2D), recrystallized from chloroform to give (4.5 g, 85.6%).M.p.92-94°C.IR,nmax/ cm-1 3340 (NH), 1721 (O-C=O), 1672 (N-C=O).
2-Benzamidoethylbenzoate (2E)
2-Benzoamido benzoic acid (2C) was esterified with ethanol and H2SO4 to give white crystalline 2- bezamidoethylbenzoate (2E)as white crystalline, recrystallized from chloroform to give (3.7 g.,66 %): M.p.94-98 °C.. IR,nmax/ cm-1 3340 (NH), 1720 (O-C=O), 1672 (N-C=O).
General procedure for preparation of hydrazides 3a and 3b
The esters 2(A-D)(3 g.) dissolved in ethanol (80 ml), added to it dropwise a hydrazine hydrate 64% (5ml.) and the mixtures were heated under reflux on a water bath 90°C (20 hr. for 2(A-C) and 12 hr for 2D. The reaction monitored by TLC. The solvents were evaporated under reduced pressure to give white solids which were washed with a small quantity of ether ( fewmls) to give corresponding crystalline products:
2-aminobenzoichydrazide (3a): White crystalline (2.4 g. from 2A and 2.7 g. from 2B,90%): M.p. 119-122°C; IR,nmax/ cm-1 3333 (NH), 2626 (SH), 1631 (N-CO), 1610 (N-N-C=O), 1382 (C=S). UV ,lmax 192, 195, 208, 214, 216, 248, 326, loge 0.539, 0.617, 2.814, 3.077, 3.039, 1.451, 0.68 respectively. 1H-NMR.δH 11.2 (s, 1H,NH),7.9-6.2 (m.4H,Har), 5.42( s, H, NH), 4.1 ( d, 2H, NH2), 3.3 (SH). 13C_NMR, 205 (C=S), 170.27 (N=C-SH), 169 ( N-C-O-), [132.76, 126.89, 117.30, 116.80, 77.44, 76.60 (Car)].
2-Benzamido benzoichydrazide(3b): Whitecrystalline (2.5 g., from 2D and 2.3 g., from 2E,84%). M.p. 195°C.IR,nmax/ cm-1 3342 (NH),2626 (SH),1630 (N-C=O).1610 (N-N-C=O), 1399 (C=S),UV, 1H and 13C-NMR are similar to above.
General procedure for preparation of 5-(2’-benzamidophenylene)-1,3,4-oxadiazole-2-thiones 4(a,b) orthioles7(a,b)
The hydrazides3a, (0.26 g.0.002 mole)or3b,(1.0g., 0.004 mole) in an ethanolic solution of KOH 85% (from KOH ,0.5 g. in ethanol 80 ml), added to themdropwise CS2 (2.5 ml for 3a and 10ml for 3b) and the mixture was refluxed at 80°C for 7 to 22 hr. The bulk of the solvents removed under vacuum at 50°C, the remaining solid washed with iced diluted HCl in filter paper. The solid dissolved in a small amount of ethanol then left into the refrigerator to give fine crystalline products:
5-(2’-aminophenylene)-1,3,4-oxadiazole-2-thiones 4aor 7a:White crystalline ( 0.233 g, 70%), m.p. 210°C .IR,nmax/ cm-1 3410.2 strong and broad (free and bonded NH), 2626 (SH), 1632 (C=N), 1403 (C=S).UV lmax 240, 350 nm. loge 6.0, 6.0 respectively. NMR.δH 9.7 (s, 1H, NH oxadiazole ring), 7.1-6.65 (m, 4H, Har) ,6.2 (s,2H,NH2), 3.4 (s, 1H,SH).13C-NMR (δ/ppm): 205.54(C=S ), 162.11(C=N), 164.56, 155.4, 132.16, 129.8,126.5, 116.94, (Car).
5-(2’-benzoamidophenylene)-1,3,4-oxadiazole-2-thiones 4b or7b: White crystalline (0.90g, 78%), m.p. 278°C.IR,nmax/ cm-1,3333 strong and broad (free and bonded NH), 2626 (SH), 1502 (C=O), 1382 (C=S).UV lmax 242, 348 nm. loge 6.0, 6.0 respectively.1H-NMR(δ/ppm), 10.2 (s, 1H, NH oxadiazole ring), 7.8-6.30 (m, 9H, Har) , 6.2 (s,H,NH), 3.4 (s, 1H,SH).13C-NMR (δ/ppm): 198.56(C=S ), 164.34(C=N), 166.36-,115.23(Car)
General procedure for preparation of 2-aminophenylenethiosemicarbazides 5a and 5b
The hydrazides3a(2.0 g., 0.013 mole) and 3b (1.0 g., 0.003 mole) dissolved in ethanol (200 ml for 3a and 80 ml for 3b) added to it NH4SCN (1.7 g., for 3a and 0.85 g., for 3b). HCl (15 ml for 3a and 10 ml for 3b were added dropwise, refluxed at 80°C for 14 hr. Bulk of ethanol was removed by vacuum, the thiosemicarbazides5a and 5b were precipitated, filtered off and recrystallized from methanol to give:
2-Aminophenylenethiosemicarbazide 5a.2.4 g., 86%.M.p. 180°C.IR,nmax/ cm-1 3290 (NH), 1631 (C=O), 1404 (C=S).
2-Benzamidophenylenethiosemicarbazide5b.0.98 g., 80%.M.p. 222°C.IR,nmax/ cm-1 3233 (NH), 1620 (C=O), 1404 (C=S).
General procedure for preparation of thiadiazoles8a and 8b
The thiosemicarbazides5aand 5b( 1.0g.) were dissolved in absolute ethanol (100 ml), KOH(0.5 g) was added with an aid of strring, followed by addition of CS2 ( 1.0ml). The mixture was refluxed at a temperature 75-80°C for 5 hr., cooled and acidified with HCl to pH=1to give :
5-(2’-Aminophenylene)-1,3,4-thiadiazole-2-thiols 8a: white crystalline,(0.8 g., 80%),m.p. 167°C.IR,nmax/ cm-1 3453 (NH), 1632 (C=N), 1403 (C=S).NMR.δH 9.7 (s, 1H, NH oxadiazole ring), 7.1-6.65 (m, 4H, Hphenyl) , 6.2 (s,2H,NH2), 3.4 (s, 1H,SH).13C-NMR (δ/ppm): 205.54(C=S ), 162.11(C=N), 164.56, 155.4, 132.16, 129.8,126.5, 116.94, (Car).
5-(2’-Benzamidophenylene)-1,3,4-thiadiazole-2-thiols 8b
white crystalline,(O.78 g.,78%),m.p. 267°C. IR,nmax/ cm-1 3290 (NH), 1642(N-C=O), 1402 (C=S). NMR.δH 10.2 (s, 1H, NH oxadiazole ring), 7.3-6.85 (m, 4H, Har) , 6.5 (s,2H,NH2), 3.2 (s, 1H,SH).13C-NMR (δ/ppm): 198.88(C=S ), 165.88 (C=O), 162.45 (C=N),[160.65- 110.24, (Car)].
General procedure for preparation of 1,2,4-triazoles 9a and 9b
The thiosemicarbazides6a(1.0g., 0.005 mole) dissolved in water (100 ml). An aqueous solution of NaOH (0.42 g.NaOH, in 30 ml of H2O) was added and the mixture wax refluxed for 10 hr. Water was evaporated under vacuum, solid formed , recrystallized from methanol to give brownish crystalline:
5-(2’-Aminophenylene)-1H-1,2,4-triazole-3-thiol9a: (0.86 g., 89%). M.p. 179°C. IR,nmax/ cm-1 ,3426 (NH, free), 3223 (NH, bonded), 2600 (SH), 1631 (C=N), 1403(C=S).1H-NMR.δH 9.5 (s, 1H, NH triazole), 7.9-7.3 (m, 4H, Har) ,3.4 (s, 1H,SH).13C-NMR (δ/ppm): 204.60(C=S ), 147.9(C=Ntriazole),[130.56-116.94, (Car)].
The thiosemicarbazides6b(1.0g., 0.003 mole) dissolved in water (100 ml). An aqueous solution of KOH (0.53g.KOH, in 20 ml of H2O) was added and the mixture wax refluxed for 8 hr. Water was evaporated under vacuum, solid formed , recrystallized from ethanol to give brownish crystalline:
5-(2’-Benzamidophenylene)-1H-1,2,4-triazole-3-thiol 9b: (0.86 g., 91%). M.p. 252°C. IR,nmax/ cm-1 . 3350 (NH, free and bonded), 2604 (SH), 1664 (N-C=O),1631 (C=N),1402 (C=S).NMR.δH 10.2 (s, 1H, NH triazole), 7.6-6.68 (m, 9H, Har) , 3.5 (s, 1H,SH).13C-NMR (δ/ppm): 199.60(C=S ), 165( C=O),136.8(C=Ntriazole),[132.44-112.94, (Car)].
General procedure for preparation of 4-amino-1, 2, 4-triazoles 10a and 10b
The hydrazides3aand 3b(1.0 g., 0.007 mole and 0.003 mole respectively) dissolved in methanol (50 ml), KOH (1.86 g) was added followed by dropwise addition of CS2 (5 ml). The mixture was stirred at room temperature for 15 hr. Diethyl ether (30 ml) added and stirring continued for further 1.0 hrto give a white ppt of salt 6aand 6b.Salts 6(a,b)–individually-, without further identification, were added to it hydrazine hydrate 64% (2 ml) and the reaction mixture was refluxed at 80°C for 8 hr. Cooled to 5°C and acidified with HCl to pH=1 to give colourless crystalline, recrystallized from ethanol
5-(2’-Aminophenylene)-4-N-amino-1,2,4-triazole-3-thiol10a(1.27g., 93%),m.p.160°C;IR,nmax/ cm-1 3423 (NH), 3063 (CHar), 2473 (SH), 1622 (C=N).NMR.δH 8.9 (s, 1H, NHtriazole), 7.6-6.8 (m, 4H, Har) , 6.52 (s,2H,NH2), 3.4 (s, 1H,SH).13C-NMR (δ/ppm): 147.9(C=Ntriazole),[130.56-116.94, (Car)].
5-(2’-Benzoamidophenylene)-4-N-amino-1,2,4-triazole-3-thiol 10b (1.06 g., 80%), m.p. 225°C.IR,nmax/ cm-1 3290 (NH), 2630 (SH), 1643(N-C=O), 1624( C=N), 1398 (C=S).1H-NMR.δH 9.6 (s, 1H, NH triazole), 7.8-6.35 (m, 9H, Har) , 3.2 (s, 1H,SH).13C-NMR (δ/ppm): 202.40 (C=S ), 163( C=O),135.0(C=Ntriazole),[133.44-110.5, (Car)].
General procedure for preparation of S-nucleosides 11(a,b)- 14(a,b)
Thiols7(a,b)-10(a,b) ( 0.01 mole) and D-glucose ( 0.01 mole) were dissolved in ethanol (100 ml) added to it HCl ( 8 ml) and the mixture was refluxed at 80°C for 5-8 hr. Proceeding of the reaction was monitored by tlc. Volatile solvent removed by vacuum, the syrup or the solids remain were washed with ether to give:
5-(2’-Aminophenylene)-1,3,4-oxadiazole-2-S-glucoside (11a), yellow syrup. IR,nmax/ cm-13423 (OH), 3290 (NH), 3067 (CH), 2998 (CHar),1630 (C=N). RMN, 8.3 (s,1H,NH), 8-7.5 (m, 4H, Har),4.5(m, 4H,O-H); 3.7(m,8H, C-H),3.2(d,2H, H2-C-O).13C-NMR (δ/ppm):167( C=N), 140-120(Car), 78-72 (Csugar)
5-(2’-Benzamidophenylene)-1,3,4-oxadiazole-2-S-glucoside (11b): Yellow syrup.IR,nmax/cm-1 342O (OH), 3330 (NH), 3067 (CH), 2990 (CHar), 1632 (C=N). 1H-RMN, 12.3 (s,1H,NH), 8-7.2 (m, 9H, Har),4.0(m, 4H,O-H); 3.7(m,8H, C-H), 3.2(d,2H, H2-C-O).13C-NMR ,171 (C=O), 167( C=N), 136-118(Car), 76-60 (Csugar)
5-(2’-Aminophenylene)-1,3,4-thiadiazole-2-S-glucoside(12a):Yellow syrup, . IR,nmax/ cm-1 3360 (broad,OH and NH), 3070 (CH), 2996 (CHar), 1632 (C=N).1H-RMN, 8.6 (s,1H,NH), 7.5-6.5 (m, 4H, Har),4.2 (m, 4H,O-H); 3.57(m,8H, O-C-H), 3.5 (d,2H, H2C-O).13C-NMR: 175 and 160 ( C=N), 150 (Car), 60 (Csugar).
5-(2’-Benzamidophenylene)-1,3,4-thiadiazole-2-S-glucoside(12b): Yellow syrup,IR,nmax/ cm-11 342O (OH), 3330 (NH), 3067 (CH), 2990 (CHar), 1632 (C=N).1H-NMR, 12.3 (s,1H,NH), 8-7.2 (m, 9H, Har),4.0(m, 4H,O-H); 3.7(m,8H, C-H), 3.2(d,2H, H2-C-O).13C-NMR (δ/ppm): 169 (C=O), 164 ( C=N), 136-118(Car), 78-63 (Csugar)
5-(2’-Aminophenylene)-1,3,4-triazole-2-S-glucoside(13a: )recrystallized from ethanol: M.p. 77°C. IR,nmax/ cm–3423 (OH), 3290 (NH), 3067 (CH), 2998 (CHar), 1630 (C=N).1H-NMR, 8.4 (s,1H,NH), 7.5-7(m, 4H, Har),4.5(m, 4H,O-H); 3.6(m,8H,H-C-O), 3.3(d,2H, H2C-O).13C-NMR (δ/ppm):168 and 166 ( C=N), 137-133 (Car), 70 (Csugar).
5-(2’-Benzamidophenylene)-1,3,4-triazole-2-S-glucoside(13b): IR,nmax/ cm-1342O (OH), 3330 (NH), 3067 (CH), 2990 (CHar), 1632 (C=N). 1H-NMR, 9.5 (s,1H,NH), 7.8-6.7(m, 9H, Har),5.0(m, 4H,O-H); 3.5 (m,8H, H-C-O), 3.2(d,2H, H2-C-O).13C-NMR (δ/ppm):169 (C=O), 164 ( C=N), 134-120 (Car), 75-70 (Csugar)
5-(2’-Aminophenylene)-4-amino-1,3,4-triazole-2-S-glucoside(14a): recrystallized from ethanol, m.p. 78°C.IR,nmax/ cm-13423 (OH), 3290 (NH), 3067 (CH), 2998 (CHar), 1630 (C=N). 1H-NMR, 9.6 (s, 2H, NH2), 8.2 (s,1H,NH), 8.3-7.6(m, 4H, Har),4.3(m, 4H,O-H); 3.7(m,8H, H-C-O), 3.2(d,2H, H2-C-O).13C-NMR (δ/ppm): 169 (C=O), 164 ( C=N), 134-120 (Car), 75-70 (Csugar)
5-(2’-Benzamidophenylene)-4-amino-1,3,4-triazole-2-S-glucoside (14b): IR,nmax/ cm-1 342O (OH), 3330 (NH), 3067 (CH), 2990 (CHar), 1632 (C=N).1H-NMR, 9.8 (s,1H,NH2),8.9(s,1H,NH), 8-7.2 (m, 9H, Har),4.2(m, 4H,O-H); 3.5 (m,8H, H-C-O), 3.5(d,2H, H2-C-O).13C-NMR (δ/ppm):171 (C=O), 163 ( C=N), 134-120 (Car), 72-70 (Csugar)
The antibacterial Tests
The filter paper disc method was performed in duplicate using fresh Mueller Hinton agar medium. This agar medium was inoculated with 0.5mL of cultures containing about 106 CFU/mL. Filter paper discs (5 mm diameter) saturated with solutions of each compound (concentrations 10µg mL–1 DMSO) was placed on the indicated agar mediums. The incubation time was 24 h at 37 °C. The blank test disc with DMSO and positive reference amykacinewere used. Inhibitory activity was evaluated by measuring the diameter of clear zone observed around the disc (in mm).
Minimum inhibition concentration (MIC) Tests
Each 1 mL of the original concentration [ c ] ( 10 µg mL-1 ) in DMSO of the compounds7(a,b)-10(a,b) and 12(a,b)were diluted with DMSO for four times to1/2 [ c ], 1/4[ c ] , 1/8 [ c ] ,1/16[ c ] , and optical density was measured at 0 Hr, 18 Hr, 24 Hr and 48 Hr.
Results and Discussion
Synthesis of hydrazides3(a,b)
Esters and benzamido acid2(A-E) and the corresponding hydrazides3(a,b) are the common intermediates for the synthesis described in this work. They should be available in relatively large quantities toutilize for further synthesis. In best opportunity, 2-aminomethylbenzoate2Awas obtained by classical esterification of 2-aminobanzoic acid 1 with methanol catalysed by H2SO4to give a modest yield (12%). Since 1 showed a better solubility in ethanol, classical esterification of 1 using H2SO4 or HCl as catalysts gave a best yield of 2-aminoethylbenzoate 2B in 30% yield. Generally, the yield of both methyl and ethyl 2-aminobenzoates 2(A,B)respectively were considered very poor and unsuitable for stepwise synthesis of nucleobases7-10(a,b) (see Scheme 1). The yield percentages of esterification been raisedfirstly by using montmorillonite argil as catalyst and secondly by protecting the amino group via N-benzoylation of 1to give 2-benzamido benzoic acid 2Cprior to esterification. Methyl and ethyl 2-aminobenzoates 2(A,B)resulted from argil as catalyst gave higher yield (65%) . Similirlay2-benzamido methyl and ethylbenzoates2(D, E) werealsoproduced in (65%) yield.
Hydrazides3(a,b) were obtained in excellent yields 3a( 90%) and 3b (84%) by refluxing the corresponding esters2(A-E) with hydrazine hydrate 51% in methanol solution. Hydrazides3a and 3b characterized by IR, UV, 1H-NMR and 13C-NMR (seeexperimental).
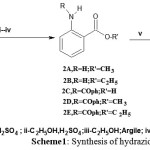 |
Scheme 1: Synthesis of hydrazides3a and 3b. |
Synthesis of nucleobases7(a, b)-10(a, b)
The synthesis of heterocyclic thiols (nucleobases)7(a,b)-10(a,b) have been obtained from the hydrazides3a and 3b into three synthetic routes A, B and C.
Route A gave rise to oxadiazoles4a and 4b, which obtained by treating –indvidually- the hydrazides3a and 3b with ethanolic KOH followed by addition of CS2 under reflux for 14 hours. IR spectra suggested the presence of keto-enoltautomers4(a,b)⇄7(a,b)detected by the presence of NH and SH bands in the region 3333 cm-1and 2626 cm-1 respectively and for C=S group in 1404cm-1. While the NMR spectra exhibited signals at 11.2 ppm and 3.3ppm for the NH and the SH respectively while 13C-NMR showed both thione at 205ppmandat170.27 ppm forthiol form (N=C-SH) .
Route B gave rise to the synthesis of thiadiazolethiols8(a, b) and triazoles9(a, b). Reaction of the hydrazides3(a, b) with ammoniumthiocyanide resulted into formation of thiosemicarbazides5(a, b) in excellent yields (86% for 5a and 80% for 5b). IR5a spectra showed a broad band cantered at 3290 cm-1 for NH, 1631 for (C=O) and 1404 for(C=S). IR5b, 3233 (NH), 1620 (C=O), 1404 (C=S).
Treatment of 5(a,b) with alcoholic KOH followed by acidification with HCl to pH=1 resulted into very good yields of thiadiazoles8a in 80% yield and 8b in 78% yield. While treatment of thiosemicarbazides5(a, b) with aqueous NaOH or KOH furnished 1,2,4-triazole thiols9(a,b) in excellent yields (89% for 9a, and 91% for 9b ).
i: CS2, KOH, Reflux; ii: NH4SCN; iii: KOH,CS2,Room temp; iv: KOH, CS2,EtOH, HCl; : NaOH, H2O; vi: NH2NH2, KOH.
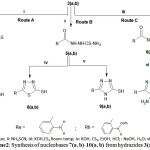 |
Scheme 2: Synthesis of nucleobases7(a, b)-10(a, b) from hydrazides3(a,b).
|
Route C dealt with synthesis of 2’-amino-1, 2, 4-triazole thiols10(a,b) which involving treatment of the hydrazides3(a,b) with CS2 in presence of KOH to give potassium salts of hydrazinic acids 6(a,b). The latters without further identification when treated with hydrazine hydrate 64% and KOH resulted the 2’-amino phenelyn-1,2,4-triazole thiols10(a,b) in very good yields (10a, 93% and 10b, 80%).
Synthesis of glycosides: 11(a,b)-14(a,b)
The second target of this work was the synthesis of S-glycosides; those been achieved by Fischer glycosidation reaction of unprotected monosaccharides with thiol groups in the presence of an acid catalyst. Thus, refluxing of nucleobases7(a,b)-10(a,b) with D- glucose they yielded S-glucosides11(a,b)-14(a,b) (see Scheme 3). Thin layer chromatography.
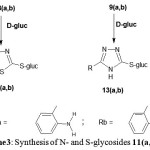 |
Scheme 3: Synthesis of N- and S-glycosides 11(a,b)-14(a,b).
|
have shown formation of one spot after each reaction with disappearance of nucleobases spots except for 14(a,b).The latter always exhibited two very close spots were difficult to separate by column chromatography. They related to mixture of S- and N-glucosides. No further work was done to separate these mixtures. IR and NMR measurements and melting points supported the tlc for formation of S-glycosides 11(a,b)-14(a,b).
Biological Tests
Nucleobases7(a,b)-10(a,b) and one type of S-glucosides namely 5-(2’-amino and 2’-benzamidophenylene)-1,3,4-thiadiazole-2-S-glucosides (12a,b)were tested against representative microorganisms. Two Gram-positive bacteria Staphylococcus aureusATCC 25923andBacillus cereusand two Gram-negative bacteria Escherichia coli ATCC 25924, PseudomonasaeruginosaATCC 27835.The filter paper disk method (NCCLS) 25was employed in duplicate for in vitro study of antibacterial effects andamykacinewas used as a positive reference. The inhibitory effects summarized in Table 1.
Table 1: Inhibition ofmicroorganisms by compounds 7(a,b)-10(a,b)and 12(a,b)in mm
|
Compounds.٭ |
Gram positive |
Gram negative |
||
|
S.aureus |
B. cereus |
E.coli |
P.aeroginosa |
|
|
7a |
0 |
20 |
31 |
11 |
|
7b |
10 |
11 |
12 |
0 |
|
8a |
19 |
20 |
0 |
33 |
|
8b |
21 |
0 |
16 |
19 |
|
9a |
0 |
16 |
13 |
12 |
|
9b |
18 |
0 |
17 |
20 |
|
10a |
11 |
19 |
13 |
21 |
|
10b |
19 |
25 |
0 |
11 |
|
12a |
14 |
10 |
||
|
12b |
0 |
0 |
||
|
Amykacine |
20mm |
19mm |
22mm |
0 |
*concentration 10 µg mL-1
From Table 1, it is shown that almost all synthetic nucleobases7(a,b)-10(a,b)and glycoside 12a exhibited various effect on the representative bacteria. It seemed that it is difficult to generalize whether there are differences in activity between the aminophenylenenucleobases7a-10a and their benzamido derivatives 7b-10b. There are only some cases where the effect exceeded the positive reference amykacine as inoxadiazole7a on G(-)E.coli , activity of thiadiazole8a upon G(+)B. cereusand activity of all tested nucleobases7(a,b)-10(a,b) on G(-)P. aeraginosa. 5-(2’-Aminophenylene)-1,3,4-thiadiazole-2-S-glucoside (12a) exhibited a moderate effect upon G(+) S. aureusand G(-) E. coli ,while its benzamido derivative 12b showed no effect toward the two organisms.
Minimum inhibition concentration (MIC) test were performed only on compounds showed an effect in initial concentration 10 ug.mL-1. Testswere done in duplicate and the average results shown in Table 2.
Table2: Minimum inhibitionconcentrations (MIC)٭
| Com. |
S. aureus |
B. cereus |
E.coli |
P. aeroginosa |
||||||||||||||
|
µg mL-1 |
µg mL-1 |
µg mL-1 |
µg mL-1 |
|||||||||||||||
|
i |
ii |
iii |
iv |
I |
ii |
iii |
iv |
i |
ii |
iii |
iv |
i |
ii |
iii |
iv |
|||
|
7a |
– |
– |
– |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||
|
7b |
+ |
+ |
– |
– |
+ |
+ |
+ |
– |
+ |
+ |
– |
– |
– |
– |
– |
– |
||
|
8a |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
+ |
+ |
+ |
+ |
||
|
8b |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
+ |
+ |
+ |
– |
+ |
+ |
+ |
– |
||
|
9a |
– |
– |
– |
– |
+ |
+ |
+ |
– |
+ |
+ |
+ |
– |
+ |
+ |
+ |
– |
||
|
9b |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||
|
10a |
+ |
+ |
– |
– |
+ |
+ |
+ |
– |
+ |
+ |
– |
– |
+ |
+ |
+ |
+ |
||
|
10b |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
– |
+ |
– |
– |
– |
||
|
12a |
+ |
+ |
+ |
+ |
+ |
+ |
– |
– |
||||||||||
|
12b |
– |
– |
– |
– |
– |
– |
– |
– |
||||||||||
|
i =1/2.C |
ii=1/4.C | iii=1/8.C | iv=1/16.C ٭ (C= initial concentration = 10 µg mL-1) | |||||||||||||||
Conclusion
Esterification and benzamidation of 2-amino benzoic acid proved to be synthetically valuable. 2-Benzamido benzoic acid exhibited better solubility in alcohols which lead to higher yield of esters and ultimately give higher yield of hydrazides3(a,b). Since the hydrazides3(a,b) were considered the key intermediates for the synthesis of nucleobases 7-10(a,b) and glycosides11-14(a,b).Also some differences observed in biological activity caused bybenzamido derivatives of 2-amino benzoic acid particularly in synthesizingnucleobases and S-glucosides12(a, b).
Acknowledgments
Thanks to Mrs. Karima Abed for typing and arranging the manuscript.
References
- CleavesIIH. J.2011, 1455,Springer Berlin Heidelberg.doi: 10.1007/978-3-642-11274-4_1371.
- Fiandaca M.J.; Hyldig-Nielsen J.J.; Gildea B.D.; Coull,J.M. Genome Res.2001, 11: 609–613.
- Hafez H.N.; Hussein H.A.R.; El-Gazzar A.B.A.Eur. J. Med. Chem.2010,45(9):4026–4034.
- Mohamed A. M.; Al-Qalawi H. R. M.; El-Sayed W.E.;Arafa W. A. A.ActaPoloniae Pharm. Drug Res.2015,72: 307-331.
- Khalil N. S.A.M.Carbohydrate. Res.2006,341(13): 2187–2199.
- Aderiye B.I.; Oluwole O. A. Agri. Biol. Sci. J.2015, 1(5): 206-216. http://www.aiscience.org/journal/absj.
- AzabME.; RizkS.A.; Mahmoud N. F.Chem. Pharm. Bull.2016, 64(5): 439-450. doi: org/10.1248/cpb.c15-01005.
- Sharonova T.; Baykov S.; Dorogov M.IRJPAC.2016,10(3): 1-5. doi: 10.9734/IRJPAC/2016/22429.
- Shigeta S.; Mori S.; Baba M.; Ito M.; Honzumi k.; Nakamura K.; OshitaniYNumazaki H.; Matsuda A and Obara T. Antimicrob.Agents Chemother.1992, 36(2):435-439. doi: 10.1128/AAC.36.2.435.
- Krajczyk A.; Kulinska K.; Kulinski T.; Hurst B.L.; Day C. W.; Smee D.F.; Ostrowski T.; Januszczyk P.; ZeidlerJ.Antiviral Chem. Chemotherapy2014, 23:161-171.doi: 10.3851/IMP256.
- Kini GD.; RobinsRK.; Avery T.L. J.Med. Chem. 1989, 32 (7):1447–1449.doi: 10.1021/jm00127a008.
- WitkoviskiJT.;Robins RK.J. Org. Chem. 1970,35(8):2635-2641.doi: 10.1021/jo00833a033.
- CipollaL. ; PalmaA.; La Ferla B.;Nicotra F. J. Chem. Soc., Perkin Trans. 2002, 1.2161-2165.doi: 10.1039/B206623
- Brito-Arias M.“Synthesis and Characterization of Glycosides”.Springer international publishing, Swistzerland,.2016, 81-168.doi10.1007/978-3-319-32310-7_2.
- Schmidt R. R.;Angew.Chem.International.1986,25(3): 212–235, doi: 10.1002/anie.198602121.
- NicolaouK. C. ;Chucholowski A.;Dolle R. E.; Randall J. L. J. Chem. Soc., Chem. Commun.1984, 1155-1156.doi: 10.1039/C39840001155.
- Almasirad A.; Tabatabai SA.; Faizi M.; Kebriaeezadeh A.; Mehrabi N.; DalvandiA.;Shafiee,Bioorg.Med.Chem.Lett.2004, 14(24):6057-6059.doi.org/10.1016/j.bmcl.2004.09.072: 6057.
- AmaroucheL.; TaiebBrahimi F.; Othman A. A.J.Chem. Pharm. Res. 2016, 8(4): 896-900. ISSN: 0975-7384.
- OthmanAA.; Kihel M.; Amara S. ArabJ Chem. 2014,doi.org/10.1016/j.arabjc.2014.09.003 (references theirin).
- S. M.,Vaughan M. D., Withers S. G., Current Opinion in Chemical Biology. 2006, 10(5): 509–519. doi:10.1016/j.cbpa.2006.07.015.
- Kumar S.;Singh D.; Dutta M.J. Oilseed Brassica.2014, 5(2): 87-95.
- JohnsonS.;Tanaka F. Org. Biomol. Chem.2016,14:259-264.doi: 10.1039/C5OB02094H.
- Zavareh E. R.;Hedayati M.;RadL. H.;Shahhosseini S.; Faizi M.;TabatabaiSA.Iran J PharmRes. 2014, 13(Suppl) :51-9.
- ChavhanNM.;Badadhe PV.;ShelkeSN.Int J InnovResSciEngTechnol.2015, 4:417- 421. doi: 10.15680 /IJIRSET.2015.0402027.
- Dallal MMS, Doyle MP, Rezadehbashi M, Dabiri H, Sanaei M, Modarresi S, Bakhtiari R, Sharifiy K, Taremi M, Zali MR, Sharifi-Yazdi MK :Food Control . 2010, 21: 388–392. doi: 10.1016/j.foodcont.2009.06.001

This work is licensed under a Creative Commons Attribution 4.0 International License.









