Synthesis, Properties and Hydration Characteristics of Novel Nano-Size Mineral Trioxide and Tetracalcium Phosphate for Dental Applications
M. M. Radwan1*, H.K. Abd El-Hamid1 and Shaymaa M. Nagi2
1Ceramics Department, National Research Centre, National Researchcentre, Giza. Egypt. 33 El Bohouthst. (former El Tahrirst.) - Dokki- Giza- Egypt- P.O.12622.
2Restorative and Dental Materials Department, National Research Centre, Egypt.
Corresponding Author E-mail: mmahmoudradwan@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320516
Nano particles made of pure single phase Mineral trioxide [tricalcium silicate (C3S)] and Tetracalcium phosphate (TTCP) bio ceramics were prepared via solid state reaction at high temperature. Setting times, compressive strength, pH, XRD, FTIR and scanning electron microscopy (SEM) were investigated. Cytotoxicity and antibacterial activity of both C3S and TTCP to human gingival fibroblasts were studied. Both C3S and TTCP compounds undergo hydration reaction when mixed with distilled water. A delayed setting time and very poor mechanical properties for TTCP were detected compared to those of C3S phase. The nano-structure C3S bio ceramic was found to be completely safe to BHK cellscompared with TTCP. The prepared C3S and TTCP pastes were applied to the demineralized dentin specimens for seven days. The ultramorphology of dentin revealed that both C3S and TTCPare successful in dentinal tubules occlusion, thus they are beneficial for dental application in the treatment of teeth hypersensitivity.
KEYWORDS:Tricalcium silicate; Tetracalcium phosphate; Dentinal tubules; Teeth hypersensitivity; Cytotoxicity
Download this article as:| Copy the following to cite this article: Radwan M. M, El-Hamid H. K. A, Nagi S. M. Synthesis, Properties and Hydration Characteristics of Novel Nano-Size Mineral Trioxide and Tetracalcium Phosphate for Dental Applications. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Radwan M. M, El-Hamid H. K. A, Nagi S. M. Synthesis, Properties and Hydration Characteristics of Novel Nano-Size Mineral Trioxide and Tetracalcium Phosphate for Dental Applications. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21947 |
Introduction
Exposure of dentin to the oral environment leads to a painful clinical condition known as Dentinal hypersensitivity (DH)[1]. Attrition, abrasion, erosion, abfraction or gingival recessions are themain reasons for dentin exposure [2]. The movement of the dentinal fluid stimulates the nerves and causes pain. So, the concept of tubule occlusion as technique of dentin desensitization is a valid relationship to the hydrodynamic theory [3]. In order to improve this, there have been known a method of coating with a polymeric material and a method of applying two materials alternately to make an inorganic salt deposit to form a physical barrier, thereby closing dentinal tubules. However, these methods have a problem thatis a cover is worn by a toothbrush or the like to be broken easily because only a surface or a shallow portion near openings of dentinal tubules are covered. Moreover, although being a material with high biocompatibility, there is another problem with it that application of the material makes a plaque adhere, causing inflammation and root caries[4].Several bio-ceramic materials have been frequently used in medical field for more than four decades to restore diseased or damaged hard tissue[5].
Increasing interest has been dedicated to calcium phosphate materials due to the similarity of its chemical composition to that of bone mineral. Thus, calcium phosphate ceramics (CPC) which belong to the second generation of biomaterials were expected to have superior biological properties. These bioactive ceramics include mainly hydroxyapatite Ca10(PO4)6(OH)2(HA), tetra-calcium phosphate (Ca4(PO4)2O, tricalcium phosphate Ca3(PO4)2(TCP) and biphasic calcium phosphates (BCP, a mixture of HA and TCP) [6-8].
Tetracalcium phosphate (Ca4(PO4)2O, TTCP) showed good properties for several dental applications including root canal filler/sealer[9,10] and base applications[11]. It was stated that the X-ray diffraction patterns of both TTCP and hydroxyapatite (HA) are identical and the two salts are structurally related, so, it is difficult to detect TTCP in the presence of HA[12, 13]. TTCP is the only calcium phosphate phase with a Ca/P ratio greater than stoichiometric hydroxyapatite (HA), and is formed above 1300oC in CaO-P2O5 system. At a temperature range 1000-1200oC the compound is metastable, therefore the synthesis of the TTCP phase-pure requires either rapid quenching or the absence of moisture to prevent decomposition to HA [14, 15].
More recently dental materials based on tricalcium silicate (C3S) have been developed and synthesized in the laboratory from highly pure raw materials unlike the portland cement in mineral trioxide aggregate (MTA) [16 -20].C3S has been used with and without additives as bone cement[21, 22] and as a posterior restorative material [23]. It was stated that the pure C3S has good bioactivity [24], the ability to be coated with HA[25, 26] and maintenance of the bone-biomaterial interface once implanted[27]. Also C3S has been proved to have adequate physical properties[21], shorter setting time than MTA, and acceptable in vitro degradability (the ability for the implanted cement to be replaced by natural tissue) [27].
TTCP (4CaO.P2O5) can also develop desirable physical properties as a result of undergoing hydration reaction similar to the hydration of 3CaO.SiO2 (formation of hydrated compounds and calcium hydroxide). The hydration reactions of C3S and TTCParegiven in Eq. (1&2): [28]
3CaO.SiO2 + ~5.3H2O → ~1.7CaO.SiO2.4H2O + ~1.3Ca(OH)2 (1)
3[4CaO.P2O5] + 3H2O → Ca10(PO4)6(OH)2 + 2Ca(OH)2 (2)
The reaction products are stoichiometric HA and calcium hydroxide (CH) [29].
The above mentioned reactions have been suggested as a mean to fill root canals in teeth[30]. The high pH associated with the formation of calcium hydroxide is not considered problematic as it is essential to destroy any bacterial colonies that may be present.
Thus the aim of this study was to assess the ability of two experimental pastes, one based on C3S and the other based on TTCP to close the dentinal tubules. In addition to studying the physico-mechanical and chemical properties by measuring setting times, compressive strength, pH of immersion solution, X-ray diffraction analysis, infra-red spectroscopy and scanning electron microscopy. The evaluation of cytotoxicity and antibacterial activity of both synthesized compounds to human gingival fibroblasts were also carried out.
Materials and Methods
Materials preparation and characterization
C3S phase (27-30 nano-meter) was synthesized by firing molded cubes of 3:1 CaO: SiO2 molar ratio, using ultra pure limestone and quartz (99.6% SiO2), in the presence of 0.5% boric acid at 1000º C for 2- hours [31]. The product was ground, remolded using carbon tetrachloride and fired at 1450º C for 2-hours. The firing process was repeated until completion of the reaction. The end product was checked for the presence of free lime.
The most common method for the preparation of TTCP is limited to solid-state reaction at high temperature [32-35]. This method is mainly based on mixing mixtures of calcium carbonate (CaCO3) and monocalcium phosphate monohydrate (Ca(H2PO4).H2O) in a molar ratio of 3:1 in n-heptane for 16 h. The slurry was then filtered, dried at room temperature and heated to 1450-1500oC for 6-12h and rapidly quenched to room temperature according the following chemical reaction:
3CaCO3 +Ca(H2PO4)2.H2OCa4→(PO4)2O + 3CO2 + 3H2O
The resulting powder was milled in n-heptane for 8h, dried at 100oC, and kept under vacuum to avoid hydration[36].
The two synthesized powders wereexmained by X-ray diffraction (XRD) to verify the identity of the synthesized compound using a copper target with radiation; wavelength=1.54-nm, X-ray was generated at 40-KV with a current of 2-5-Ma. The scanning speed was 1o/minute. The final materials were finely ground in agate mill until the desired particle size given in (Fig. 1,2) that was evaluated by Transmission Electron Microscope (TEM) (JEM-1230) at 100Kv.
 |
Figure 1: XRD spectral analysis (2-Theta-scale) of the two synthesized basic constituents of the experimental cements (a) C3S – (b) TTCP. |
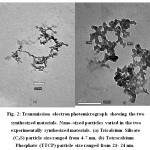 |
Figure 2: Transmission electron photomicrograph showing the two synthesized materials. Nano–sized particles varied in the two experimentally synthesized materials. (a) Tricalcium Silicate (C3S) particle size ranged from 4-7 nm, (b) Tetracalcium Phosphate (TTCP) particle size ranged from 21- 24 nm. |
Physical characteristics of experimental C3S and TTCP
Setting time
Five Scoops of the powder were mixed with five drops of distilled water to fill a mold of dimensions; 10 mm + 0.1 mm height. Mixing was done on a clean glass slab with a clean spatula, for thirty seconds. Each mold was placed on a glass plate 1-mm thick X 25 mm wide X 75 mm long and filled with the tested C3S andTTCP pastes to a level surface. After 120 + 5 seconds from the beginning of mixing, the assembly was placed in a cabinet maintained at 37+ 1oC temperature and 95% relative humidity. The Gilmore needle (having a flat end diameter of 2 mm + 01mm) was carefully lowered on to the horizontal surface of the material. Indentations were repeated at 30-second intervals until the indenter fails to make a complete circular indentation in the cement. The indenter was wiped clean between indentations.
Compressive strength
The prepared pastes of C3S and TTCPwere poured into in plastic moulds of 1/2 inch cylinder. The paste was placed in the mould into two approximately equal layers. Each layer was compacted and pressed along the surface of the mould until homogenous specimen was obtained. After the top layer was compacted, the moulds were then vibrated manually for two minutes to remove any air bubbles and to have a better compaction of the paste. The surface of the paste was smoothened with the aid of thin edged trowel. Immediately after moulding, the samples were cured in a humidity chamber at 100% relative humidity at a constant temperature of 35oC for 24 hours. At the end of the moist curing period the samples were demoulded and then cured under the distilled water until the time of testing at a constant temperature of at 37+ 1oC temperature such as 1, 3, 7and 28days. The compressive strength test [37] was carried out on three cubes of each case of the hardened cement pastes. The mechanical behavior of compressive strength machine was analyzed with a LLOYD Instrument, Model LR 10K.
PH of immersion solution
To test in vitro pH variation of the two types cement pastes (TTCP & C3S) in distilled water. 1/2 inch cylinderof each type of cements was directly poured into a 25 ml beaker and then 15 ml of distilled water was injected into the container to cover the paste[38]. The test samples were stored in a 35oC, 100% humidity water bath. After 3, 7 and 28 days of immersion, all solutions were filtrated and its pH value was measured using an electrolyte-type pH meter.
X-ray diffraction of the two prepared cement pastes
Selected hydrated samples were investigated by X-ray diffraction (XRD) to verify the identity of the hydrated compounds using a copper target with radiation; wavelength= 0.154 nm, X-ray was generated at 40 kV with a current of 2-5-mA.
ATR/FTIR Spectroscopy
The hydrated specimens of the two synthesized materials were investigated using IR spectra. IR spectra were recorded onJASCO 4600 model FTIR spectrometer.
Scanning electron microscopy (SEM)
The morphology of the hydrated dry samples of the two specimens were investigated using scanning electron microscopy (SEM), with the aid of (Jeol JSM-T20) with coating of a thin layer of gold using sputter coater S150A.
Cytotoxicity and antibacterial Activity
Experimental C3S and TTCP were formulated as blocks. Then were sterilized by ethylene oxide gas, (Arab medical equipment company (AMECO). Sterilized disks were soaked in Hanks Balance salt solution (HBSS) for 7 days as 10mg/ml. Extract was dispensed to pre cultured fibroblast cell line Baby hamster kidney (BHK) seeded as 2×105 /ml. C3S and TTCP extracts were 2 fold serially diluted post decanting the growth medium from pre-cultured plates. Treated cells were examined microscopically using inverted microscope (Hund–Germany) for detection of cellular changes and pathological changes. C3S and TTCP extracts treatment medium was decanted 24 hrs post treatment. Residual live cells were counted using MTT stain for detection of cytotoxicity. Residual live cell count was determined based on the following equation[39, 40]:-
Viab.% = Mean OD of Test sealer / Mean OD of control X100
Indirect contact test
The methodology used was adapted from Zhang et al. (2010)[41]. The experimental C3S and TTCP pastes were loaded to sterile disks of 5 mm diameter and 1 mm thickness. Disks were dried in sterile Petri dishes at 370C. St. Faceles strain was spread on solid TSB. Plates were maintained at +40C for an hour for pre-diffusion of the extracts and then incubated at 370C for 24 hrs under aerobic conditions. The inhibition zones around each one of the wells were then measured in millimeters using a digital paquimeter.
Direct Contact Method
The pure cultures were grown on 5% sheep blood plus brain heart infusion (BHI) agar plates (Oxoid, Basingstoke, UK). After 24 hrs, the colonies were suspended in tubes containing 5 mL of Tryptic soya broth; TSB, [Oxoid- USA]. The cell suspension in each tube was adjusted spectrophotometrically at 800 nm (O.D.800) to match the transmittance of (equivalent to 0.5 McFarland scale =1.5 x 108 CFU).
The following systems were tested: antimicrobial activity was checked hourly, using the spectrophotometer, up to the 18th hrs for TSB + bacterial inoculum + in micro titer plate serially diluted test sealers. Wells containing only microorganisms were used as positive controls. Bacterial system (St. Fecales) was used as (50 µL). Plates were incubated for 18-24 hrs under adequate gaseous conditions, in order to check microbial growth. Optical density represent bacterial growth inhibition was read using ELISA plate reader[42-46].
Dental application of the experimental C3S and TTCP
Preparation of dentin specimens.
Dentin specimens were prepared from extracted caries-free human third molars and stored in 0.1M phosphate buffered saline (PBS) solution at 4°C for no longer than two months prior to use. Occlusal enamel was removed using diamond saw under copious water coolant to expose the dentin. Then dentin surface was ground with water-lubricated abrasive silicon carbide paper to obtain standardized remaining dentin thickness of 2mm. Finally the dentin specimens were polished with pumice and water. Then the dentin specimens were etched with 37% phosphoric acid (pH 0.6) for 15 seconds and rinsed to open the dentinal tubules, simulating hypersensitive dentin.
Preparation of C3S and TTCP pastes for treatment of dentin specimens
The prepared C3S and TTCP powder were mixed with distilled water. The mixtures were stirred for 1 minute to form homogeneous pastes. Then each paste was applied to the demineralized dentin specimens with a cotton swab and left in place for 3 minutes. The experimental pastes were applied everyday for seven days with application time three minutes each[47]. After each application process, the specimens were washed with distilled water, then stored in artificial saliva [(in mM/L) was CaCl2 (0.7), MgCl2 6H20 (0.2), KH2PO4 (4.0), KCl (30), NaN3 (0.3) and HEPES buffer (20), the pH was 7.4] [48]. Artificial saliva was refreshed every 24 hours. After 7 days, the specimens were removed from the artificial saliva and washed thoroughly with distilled water [49].
Scanning electron microscope evaluation of dentin specimens
The morphology of dentin specimens were observed using SEM [Model Quanta 250 FEG (Field Emission Gun)] at different magnifications 6000X and 1200X. All dentin discs were air-dried at room temperature and gold coated before analysis. Images of the dentin surface were taken following treatment with phosphoric acid, then after the application of each experimental paste following 7 days incubation in artificial saliva [50-53].
Statistical analysis
Data were presented as mean, standard deviation (SD) and standard error (SE) values. Data were explored for normality using D’Agostino-Pearson test for normal distribution. Setting Time, pH and compressive strength (MPa) showed a normal distribution. One way-ANOVA have been used to study the effect of different periods. Tukey’s post-hoc test was used for pair-wise comparison between the means when ANOVA test is significant. Independent t-test used to compare between different tested materials. Significant level set at p≤0.05. Statistical analysis was performed with IBM® SPSS® (SPSS Inc., IBM Corporation, NY, USA) Statistics Version 23 for Windows.
Results
Setting time
Table 1.and Fig. 3 shows themean and standard deviation (SD) of setting time for C3S and TTCP pastes. The data reveals that the setting time of C3S is statistically significant lower than TTCP when mixed with distilled water. This has a great importance in case of using these materials in dental biomedical applications as the relatively fast curing and good mechanical properties in case of the silicate cement comparing to that of calcium phosphate which will minimize the possibility of fluid leakage.
Table 1: Mean and standard deviation (SD) for different tested materials.
|
Materials |
p-value |
||||
|
C3S |
TTCP |
||||
|
Mean |
SD |
Mean |
SD |
||
|
Setting time |
76.00 |
1.58 |
92.00 |
2.24 |
≤0.001* |
Means with the same letter within each row are not significantly different at p=0.05
Significant, NS=Non-Significant*=
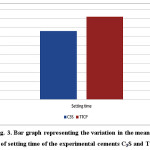 |
Figure 3: Bar graph representing the variation in the mean values of setting time of the experimental cements C3S and TTCP. |
Compressive strength
The mechanical properties were studied by determining strength values of the hydrated pastes at different curing ages. The results showed that the calcium phosphate (TTCP) didn’t show any mechanical properties after immersing under distilled water and a full distortion of all immersed cubes has been observed. The strength values of the calcium silicate (C3S) cement given in Table 2. and Fig. 4. Results revealed a statistically significant increase in the compressive strength values (MPa) with the curing age from 1 and up to 28 days.
Table 2: Mean and standard deviation (SD) of Compressive strength (MPa) of C3S
|
Time |
p-value |
|||||||||
|
1 Day |
3 Days |
7 Days |
28 Days |
|||||||
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|||
|
Compressive strength (MPa) |
C3S |
7.06a |
1.02 |
14.15b |
1.65 |
27.45c |
1.09 |
37.19d |
1.01 |
≤0.001* |
Means with the same letter within each row are not significantly different at p=0.05.
*= Significant, NS=Non-Significant
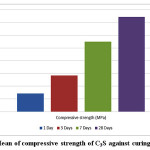 |
Figure 4: Mean of compressive strength of C3S against curing time. |
pH of immersion medium
Mean and standard deviation (SD) of the pH for different tested materials presented in Table 3 and Fig. 5. Results revealed that the pH values in case of C3S samples statistically significantly increase from 3 days curing period up to 7 days and statistically significantly decrease from 7 to 28 days curing periods. There was non statistical significant difference between pH values at 3 and 28days. For the pastes prepared from TTCP bio-cement the pH values showed a significant increase from 3 up to 28 days curing periods. Comparing both C3S and TTCP at each curing periods; the results revealed statistically significant difference between then. The pH values for C3S samples are statistically higher than those for TTCP samples except for 28 days the difference was not significant.
Table 3: Mean and standard deviation (SD) of pH
|
Materials |
p-value |
|||||
|
C3S |
TTCP |
|||||
|
Mean |
SD |
Mean |
SD |
|||
|
pH |
3 Days |
8.27a |
0.01 |
7.52a |
0.06 |
≤0.001* |
|
7 Days |
8.37b |
0.01 |
8.18b |
0.06 |
≤0.001* |
|
|
28 Days |
8.27a |
0.01 |
8.33c |
0.06 |
0.044 NS |
|
|
p-value |
≤0.001* |
≤0.001* |
||||
Means with the same letter within each column are not significantly different at p=0.05.
*= Significant, NS=Non-Significant
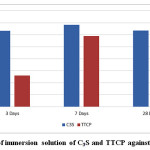 |
Figure 5: PH of immersion solution of C3S and TTCP against curing time. |
X-ray diffraction of C3S and TTCP pastes
Investigation of XRD data given in Figs.6 &7, indicates that by increasing curing periods, there is a diminishing trend in intensities of all characteristics peaks of the anhydrous C3S and TTCP phases. The XRD data of C3S (Fig.6) reveals an overlapping of those peakcharacteristicof fibrous gel like CSH over those of the unhydrous phase. The main XRD peak height of Ca(OH)2 at 2θ=18 decreases from 1 to 28 days curing period. The XRD graphs given in Fig. 7 showed a slight increase in peak intensities for both hydroxapatite and Ca(OH)2with curing age from 1 up to 28 days.
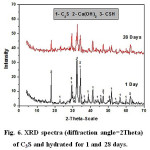 |
Figure 6: XRD spectra (diffraction angle=2Theta) of C3S and hydrated for 1 and 28 days. |
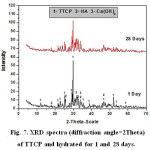 |
Figure 7: XRD spectra (diffraction angle=2Theta) of TTCP and hydrated for 1 and 28 days. |
Infraredspectroscopy
The FTIR spectra of samples of the two synthesized materials cured for 1, 3, 7 and 28 days are given in Figs. 8&9. The IR spectra of calcium silicate (Fig 8) indicates a decreasing trend in almost all band intensities of the remaining anhydrous phase (C3S-alite) at ≈ 500 (cm-1) and Si-O stretching vibration with curing period. On the other hand, there is a little increase in those characteristic for the hydrated calcium silicate compounds (at 445,815,950 cm-1 ,and incorporated ν2H2O and OH– at 1630&3365 cm-1 and 2500&3700 cm-1, respectively, corresponding to the main hydration product C-S-H compound of C3S phase.The IR bands characteristic for Aragonite (ν2,ύ3CO32 antisymmetric stretching) at 850, 1490 and 1460 cm-1 is formed due to the possible carbonation of the hydrated compounds by the action of CO2 present the atmosphere. Fig. (9) which represents the IR charts of tetra-calcium phosphate material (TTCP) reveals a slow rate of hydration reaction of phosphate cement that is emphasized by the minute diminishing trend of IR bands for the phosphate located at 1030 cm-1(Antisymmetric stretching) and 600-560 cm-1(amorphous calcium phosphate) and also the OH stretching-very broad band at 490-3650-2500 to 3700 cm-1 together with those of the incorporated molecular water in all hydrated compounds (at 1635 and 3365 cm-1).
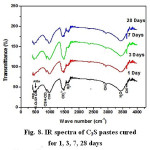 |
Figure 8: IR spectra of C3S pastes cured for 1, 3, 7, 28 days
|
 |
Figure 9: IR spectra of TTCP pastes cured for 1, 3, 7, 28 days. |
Scanning electron microscopy (SEM)
Investigation of the micrographs given in Fig.10 of C3S sample (a) and TTCP sample (b)cured for 28 days in distilled water indicates that the morphological features of the two samples are completely different. Fig. 10 (a) which represents the C3S showed a dense and closed structure of fibrous calcium silicate hydrate crystals deposited around and on the surface of the anhydrous particles of C3S phase and filling the pores between such particles. Plates of Ca(OH)2 could not be detected clearly due to the deposition of such huge amount of the CSH fibrous. The micrograph of the TTCP sample (b) shows a much more porous structure than that in Fig.10 (a). Platelets of hydroxyapatite (HA) were clearly detected in Fig.10 (b), they were found to be embedded in the unhydrous particles.
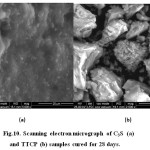 |
Figure 10: Scanning electron micrograph of C3S (a) and TTCP (b) samples cured for 28 days. |
Cytotoxicity and Antibacterial Activity
Evaluation of cytotoxicity of test materials namely TTCP and C3S to baby hamster kidney fibroblast cell line (BHK). The data revealed that C3S was completely safe to BHK cells, in the mean time a mild toxicity was noticed in case of testing TTCP recording 100 and 82 % respectively (Fig.11). Regarding the antibacterial activity against St fecalis there was no antibacterial activity detected compare to positive control using both direct and indirect methods (Fig.12). It has to be emphasized that the two test materials was soaked for only 24 hrs only which is considered very early curing period to liberate enough amount of Ca(OH)2 in the reaction medium that is may be the main reason for the negative antibacterial effect. Consequently, it is not a matter of interest to make bacterial count as the two synthesized materials have no antibacterial activity.
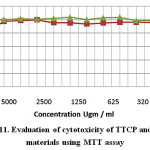 |
Figure 11: Evaluation of cytotoxicity of TTCP and C3S materials using MTT assay |
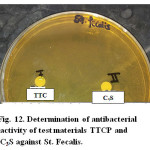 |
Figure 12: Determination of antibacterial activity of test materials TTCP and C3S against St. Fecalis. |
Results of SEM observation of the dentin specimens.
Dentin specimens were examined under SEM before and after C3S and TTCP treatment. The smear layer is obviously removed, giving patent dentinal tubules after treatment with phosphoric acid (Fig. 13a 6000x& 13b 12000x).
For the dentin specimens treated with the C3S pastes, C3S appeared to spread homogenously over inter-tubular dentin, closing most of dentinal tubules, while leaving others partially open (Fig. 14a 6000x & 14b 12000x).
For the dentin specimens treated with the tetra-calcium phosphate pastes, the pastes formed a coarse layer of deposits showing a cobblestone appearance (Fig. 15a 6000x & 15b 12000x). The dentinal tubules were occluded completely and not observable.An artificial smear layer like covering on inter-tubular dentin was observed. Agglomerates and clusters of debris showed hexagonal–rhombohedral–octahedral shape covered the entire dentin surface creating acovering of the dentin surface.
 |
Figure 13: Dentin specimens treated with phosphoric acid (a. 6000x), (b. 12000x) |
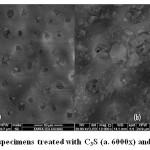 |
Figure 14: Dentin specimens treated with C3S (a. 6000x) and (b. 12000x) |
 |
Figure 15: Dentin specimens treated with TTCP (a. 6000x) and (b. 12000x). |
Discussion
Tricalcium silicate (C3S) and tetracalcium phosphate (TTCP) are similar in that they can both undergo hydration reaction resulting in desirable physical properties. Investigation of the setting property of both synthesized materials (Fig.3) indicates a relatively faster rate at which C3S sets than TTCP. The aforementioned chemical reactions (eq. 1&2) can explain the setting process of the two materials in that the precipitation of hydration products, namely, calcium silicate hydrate (C-S-H), hydroxyapatite (HA) and calcium hydroxide Ca(OH)2 immediately after mixing with distilled water is responsible for the completion of setting process. It was stated that C3S phase is the main component of MTA materials and responsible for the development of material setting and strength [16, 17]. The hydration of C3S phase starts immediately after mixing with distilled water and its paste will solidify to a hardened structure. At ambient temperature and curing age of 28 days, about 90% of the anhydrous C3S phase will be hydrated. However, in this study, the curing and hardening of nano-size (4-7nm) C3S phase were carried out in an incubator of 37oC will in turn cause an enhancement of workability, wetting volume and hydration rate [28]. Addition of bismuth oxide to both synthesized materials provides adequate radio opacity and did not have any adverse effect on the hydration process. As it was mentioned before, C3S phase is considered the main constituent in MTA material that is responsible for development of mechanical strength through formation of calcium silicate hydrate (C-S-H). The strength data given in fig.4 showed a continuous increase in the strength values with the curing ages from 1 and up to 28 days at 37oC. The slow hydration reaction rate at early ages of TTCP pastes results in a lower pH values (pH˂8) of the curing medium compared with those of C3S hydrated samples as shown in Fig. 5. The dissolution and hydrolysis of TTCP starts immediately after mixing the material with distilled water resulting in the formation of HA layer on the surface of the anhydrous particles that prevents further hydration. At 28 days curing age the less soluble HA compound will partly dissolute in the reaction medium and water molecules will reach the anhydrous TTCP particles, so, additional Ca(OH)2 will be liberated in the medium [29]. The XRD patterns of TTCP materials Fig. 7 indicate the very slow hydrolysis of TTCP materials that is emphasized by the very little change in the XRD intensities of almost all peaks for samples cured for 1 and 28 days. On the other hand, a faster hydration reaction rate of C3S phase at early hydration periods (3 and 7 days) was detected which results in a liberation of excess amounts of Ca(OH)2. This will raise the pH of the hydration medium to more alkaline (pH˃8). At later ages of hydration (28 days), huge amounts of C-S-H gel is formed as a hydrated compound of tri-calcium silicate material that will encapsulate the anhydrous C3S particles by filling the micro-pores of the hardened structure. This will prevent the penetration of water molecules needed for progressing of the hydration process [16, 17]. The XRD data of C3S samples hydrated for 1 and 28 days given on Fig. 6 may emphasize the above mentioned pH findings that is clear in lager decrease in the intensities of the anhydrous material including the free lime characteristic peak at 2θ=18° which is mainly due to the expected decrease of paste porosity. Investigation of IR spectra of the two synthesized materials (Figs.8&9) was found to be much more complicated but it can show another evidence about the difference of hydration reaction rates of both cements that is clear in a relatively considerable degree of diminishing of IR bands of the C3S anhydrous particles with the hydration reaction period together with the appearance of IR bands of the resulting hydrated compounds compared with that of TTCP cement.
Regarding the dental application of both (C3S) and (TTCP) as a desensitizing agents.SEM evaluations demonstrated that C3S coating paste was able to close most of dentinal tubules. Hydration of the C3S leads to the formation of calcium hydroxide, allowing the formation of apatite crystallites in the presence of phosphate-containing biological fluids (artificial saliva), that leads to closure of the dentinal tubules [50-52]. Similarly Suge et al, 2008 demonstrated the formation of calcium silicate-calcium phosphate precipitates after the application of ammonium hexafluorosilicate, with stable occlusion of dentinal tubules with reduced permeability [52].
A previous study by Giovanna et al., 2008 support our findings. They demonstrated that a smooth uniform and void-free silicate coating obliterating all the dentinal tubule orifices after 10 minutes of immersion into phosphate-containing salt solution (artificial saliva). Moreover they observed precipitate deposits inside dentinal tubules to a depth of 2–10 mm, in longitudinal sections in fractured samples using SEM evaluation [50].
One the other hand the TTCP paste, SEM results revealed a satisfactory occlusion of all dentinal tubules (Fig. 3a&b). Many deposits were able to occlude all dentinal tubules that were completely covered and not visible.A fine crystalline layer created an artificial smear layer like coating on inter-tubular dentin was also observed. This was due to that when TTCP particles are mixed in the presence of water, it is converted into HA slowly. The reason for this is not clear, but the following mechanism is presumed. When a dentin mineralizing agent comprising tetracalcium phosphate particles is used, an alkali metal salt of phosphoric acid in certain amounts is prepared in the presence of water. It seems t hat calcium ions produced due to dissolution of the TTCP particles react with phosphorus ions produced due to dissolution of the alkali metal salt of phosphoric acid, so that energetically stable HA is deposited. It seems that as a result a dense HA layer is formed on a surface of a dentin and HA is deposited to deep portions of dentinal tubules, so that the dentinal tubules are closed[53-57].
Conclusions
Nano-structural experimental tricalcium silicate (ultra pure single phase MTA) and tetracalcium phosphate (TTCP)bioceramics are promising materials for dental application in the treatment of teeth hypersensitivity. Tetra calcium phosphate bio ceramic (TTCP) hydrates with distilled water in a slower rate than tricalcium calcium silicate (C3S).Pastes of TTCP showed no mechanical strength, while an excellent mechanical properties of C3S pastes has been detected. Hydration of TTCP and C3S bio ceramics liberates free lime (Ca(OH)2 in the hydration medium causing increase in pH values for the two materials up to pH= 8.5. Cytotoxicity test indicated that C3S phase was completely safe to BHK cells, while, TTCP showed mild toxicity. Both experimental C3S and TTCP had no antibacterial activity against St fecalis after the first 24 hrs hydration period using both direct and indirect methods.
References
- Markowitz, K.; Pashley, D. H.; Personal reflections on a sensitive subject. J. Dent. Res. 2007;86: 292- 295.
- Pashley, D. H.; Mattews. W. G.; Zhang, Y.; Johnson, M.; Fluid shifts across human dentin in vitro in response to hydrodynamic stimuli. Arch. Oral. Biol.1996;41: 1065-1072.
- Gillam, D. G.; Orchardson, R.; Advances in the treatment of root dentin sensitivity: mechanisms and treatment principles.Endod. Topics.2006;13:13- 33.
- Aranha, A. C.; Pimenta, L. A.; Marchi, G. M.; Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Braz. Oral. Res.2009; 23: 333-339.
- Hench, L. L.; Bioceramics. J. AM. Ceram. Soc.1998; 81: 1705-1728.
- Hench, L. L.; Polak, J.M.; Third-generation biomedical materials. Science.2002; 295: 1014-1021.
- Hockin, H. K. Xu; Lisa, E. C.; Carl, G. S.; Shozo, T.; Laurence, C. C.; Premixed calcium phosphate cements: synthesis, physical properties, and cell cytotoxicity. Dental Materials. 2007;23:433-441.
- Medvecky, L.; Giretova, M.; Sopcak, T.; Preparation and properties of tetracalcium phosphate-monetitebiocement. Materials letters. 2013; 100:137-140.
- Sugawara, A.; Kusama, K.; Nishimura, S.; Nishiyama, M.; Moro, I.; Hudo, L.; Chow, C.; Takagi, S.; Histopathological reaction of a calcium phosphate cement root canal filler. J. Hard. Tissue. Biol.1995; 4: 1-7.
- Cherng, A. M.; Chow, L.C.; Takagi, S.; In vitro evaluation of a calcium phosphate cement root canal filler/sealer. J. Endod.2001; 27: 613-618.
- Dickens-Venz, S.H.; Takagi, S.; Chow, L.C.; Browen, R.L.; Johnston, A.D.; Dickens, B.; Physical and chemical properties of resin-reinfrced calcium phosphate cements. Dent. Mater.1994; 10: 100-106.
- So-Yeon, K.; Sung-Hyun, J.; Setting properties, mechanical strength and in vivo evaluation of calcium phosphate-based bone cements, Journal of Industrial and Engineering Chemistry. 2012; 18: 128-136.
- Dickens, B.; Brown, W.E.; Kruger. G.J.; Stewart, J.M.; Ca4(PO4)2O, tetracalciumdiphosphate monoxide. Crystal structure and relationships to Ca5(PO4)3OH and K3Na(SO4)2 . Acta. Cryst. 1973, B29, 2046-2056.
- Monma, H.; Goto, M.; Nakajima, H.; Hashimoto, H.; Preparation of tetracalcium phosphate. Gypsum Lime.1986; 202: 17-21.
- Brown, W.E.; Epstein, E.F.; Crystallography of tetracalcium phosphate. J Res National Bureau Standards – A. Phys. Chem.1965; 69A: 547-551.
- Camilleri, J.; Characterization of hydration products of mineral trioxide aggregate. Int. Endod. J.2008;41: 408- 417.
- Camilleri, J.; Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dental Materials.2011; 27: 836- 844.
- Wen-His, W.; Chen-Ying, W.; Yow-Chyun, S.; Cheing-Meei, L.; Feng-Huei, L.; Chun-Pin,L.;Compositional characteristics and hydration behavior of mineral trioxide aggregates. J Dent Sci.2010;5(2):53−59.
- Monteiro. B. C.; Demarchi, A. C.; de Moraes I. G.; Bernadineli, N.; Garcia, R.B.; Spangberg, L.S.; Duarte, M.A.; Presence of arsenic in different types of MTA and white and gray Portland cement. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008;106: 909-13.
- Schembri, M.; Peplow, G.; Camilleri, J.; Analyses of heavy metals in mineral trioxide aggregate and Portland cement. J. Endod. 2010; 36: 1210-1216.
- Huan, Z.; Chang, J.; Study on the physicochemical properties and in vitro bioactivity of tricalcium silicate-calcium carbonate composite bone cement. J. Mater. Sci: Mater. Med.2008; 19: 2913-2918.
- Huan, Z.; Chang, J.; Novel tricalcium silicate/monocalcium phosphate monohydrate composite bone cement.J. Biomed. Mater. Res. B Appl. Biomater.2007; 82: 352- 359.
- Laurent, P.; Camps, J.; De Meo, M.; Dehiy, J.; About I. Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dental Materials2008; 24:1486- 9.
- Chen, C.C.; Ho, C.C.; David Chen CH, Ding SJ. Physiochemical properties of calcium silicate cements for endodontic treatment .J. Endod. 2009;35: 1288-91.
- Arnon, C.; Pincha, T.; Microstructure: Surface and cross-sectional studies of hydroxyapatite formation on the surface of white Portland cement paste in vitro. Applied Surface Science.2011; 257: 8385– 8390.
- Elliott, J.C.; Structure and chemistry of the appetites and other calcium orthophosphates. Amsterdam: Elsevier; 1994.
- Jia-Cheng, L.; Jia-Xuan, L.; Qian, Z.; Wei, Z.; Wen-Qing L.; Jun-Qi, L.;Comparsion of mineral trioxide aggregate and calcium hydroxide for apexification of immature permanent teeth: A systematic review and meta-analysis. Journal of the Formosan Medical Association.2016; xx: 1-8
- Taylor, H.F.W.; Cement Chemistry, Thomas Telford, London, 1997.
- Martin, R.I.; Brown, P.W.; Hydration of tetracalcium phosphate. Adv. Cem. Res.1993;5: 119-125
- Brown, W.E.; Chow, L.C.; Combination of sparingly soluble calcium phosphates in slurries and pastes as remineralizers and cements, U.S. Patent No. 4,612,053, 1986.
- Lea, F.M.; The Chemistry of Cement and Concrete, 4th .Edn., Peter C. Hewlett, Paperback edition, London, 2004.
- Jalota, S.; Tas, AC.; Bhaduri, SB.; Synthesis of HA-seeded TTCP (Ca4(PO4)2O) powders at 1230oC from Ca(CH3COO)2H2O and NH4H2PO4. J. Am. Ceram. Soc.2005; 88: 3353-60.
- Brown, W.F.; Chow, L.C.; Dental restorative cement pastes. US patent No. 4518, 430,1985.
- Chow, L.C.; Takagi, S.; Self-setting calcium phosphate cements and methods for preparing and using them. US patent No. 5525, 148, 1996.
- Matsuya, Y.; Matsuya, S.; Antonacci, J.M.; Taagi, S.; Chow, L.C.; Akamine, A.; Effect of powder grinding on hydroxyapatite formation in a polymeric calcium phosphate cement prepared from tetracalcium phosphate and poly(methyl vinyl ether maliec-acid). Biomaterials. 1999;20: 691-7.
- Sargin, Y.;,Kizialli, M.; Telli, C.; Güler, H.; A new method for the solid- state synthesis of tetracalcium phosphate, a dental cement: X-ray powder diffraction and IR studies. J. Eur. Ceram. Soc. 1997; 17: 963-70.
- ASTM Designation: C109-80, Standard Test Methods for Compressive Strength of Hydraulic Cements, ASTM Standards, 1983.
- Nurit, J.; Margerit, J.; Terol, A.; Boudeville, P.; PH –metric study of the setting reaction of mono phosphate monohydrate/calcium oxide-based cements. J. Mater. Sci. Mater. Med.1993; 13:1007-14.
- Korzeniewski, C.; Callewaert, D. M.; An enzyme-release assay for natural cytotoxicity. J Immunol Meth, 1983; 64: 313-20.
- Decker, T., Lohmann-Matthes, M.L.; A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Meth. 1988; 115:61-9.
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill. A.J.; York, D.W.; Mechanistic investigation into antibacterial behavior of suspensions of ZnO nanoparticles against E.Coli. J. Nanopart. Res. 2010; 12: 1625-1636.
- Badawy, O.F.H.; Shafii, S.S.A.; Tharwat, E.E.; Kamal, A.M.; Antibacterial activity of bee honey and its therapeutic usefulness against Escherichia coli O157:H7 and Salmonella typhimurium infection. Rev. sci. tech. Off. int. Epiz, 2004; 23: 1011-1022.
- Johnson, T.; Case, C.; Chemical Methods of Control, adapted from Laboratory Experiments in Microbiology, Brief Edition, 4th ed. Redwood City, 1995.
- Ahmad, R.; Shahverdi, A. F.; Hamid, R.; Shahverdi, Sara M.; Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureusand Escherichia coli, MS. Nanomedicine2007; 3: 168-171.
- Morgental, RD.; Vier-Pelisser, F.V,; Oliveira, S.D.; Antunes, F.C.; Cogoand, D.M.; Kopper, P.M.P.; Antibacterial activity of two MTA-based root canal Sealers. Inter. Endod. J.2011; 1365-2591.
- Nachlas, M.; Margulies, S.; Goldberg, J.; Seligman, A.; The determination of lactic dehydrogenase with a tetrazolium salt. Anal Biochem.1960; 1: 317-26.
- Hora, B.; Kumar, A.; Bansal, R.; Bansal, M.; Khosla, T.; Garg, A.; Influence of McInnes bleaching agent on hardness of enamel and the effect of remineralizating gel GC tooth mousse on bleached enamel-An invitro study. Indi. Dent. Sci.2012; 4: 13-16.
- Pashley, DH.; Tay, FR.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvahlo, RM.; Ito, S.; Collagen degradation by host-derived enzymes during aging. J. Dent. Res.2004; 83: 216-21.
- Dong, Z.; Chang, J.; Deng, Y.; Joiner, A.; Tricalcium silicate induced mineralization for occlusion of dentinal tubules. Aust. Dent. J. 2011; 56: 175–180.
- Gandolfi, M.G.; Farascioni, S.; Pashley, D.; Giorgio, G.; Prati, C.; Calcium silicate coating derived from Portland cement as treatment for hypersensitive dentin. J.Dent.2008; 36: 565-578.
- Tay, FR.; Pashley, D.H.; Rueggeberg, F.A.; Loushine, R.J.; Weller, RN.; Calcium phosphate phase transformation produced by interaction of Portland cement component of white MTA with phosphate-containing fluid. J. Endod. 2007; 33:1347-51.
- Suge, T.; Kawasaki, A.; Ishikawa, K.; Matsuo, T.; Ebisu, S.; Ammonium hexafluorosilicate elicits calcium phosphate precipitation and shows continuous dentin tubule occlusion. Dent. Mater. 2008; 24:192–8.
- Hashimoto, T.; Sugiura, M.; Ishihara, S.; Kawashima, M.; Okada, K.; Patent Application Publication (10) Pub. No.: US 2013/0251767 , Pub. Date: Sep. 26, 2013.
- Wenjuan, L.; Dong, Z.;Zhiguang, H.;Chengtie, W.; Jiang, C.; Novel tricalcium silicate/magnesium phosphate composite bone cement having high compressive strength, in vitro bioactivity and cytocompatibility. Acta Biomaterialia.2015; 21: 217–227.
- Setbon, H.M.;Devaux, J.; Iserentante, A.; Leloupa, G.; Available online at www.sciencedirect.com Influence of composition on setting kinetics of newinjectable and/or fast setting tricalciumsilicatecements. Dental materials.2014;30: 1291–1303.
- Chia-Tze, K.;Tsui-Hsien, H.; Yi-Jyun, C.; Chi-Jr, H.; Chi-Chang, L.; Ming-You, S.; Using calcium silicate to regulate the physicochemical and biological properties when using β-tricalcium phosphate as bone cement. Materials Science and Engineering, C.2014; 43: 126–134.
- Camilleri,J.; François, S.; Denis D.; Investigation of the hydration and bioactivity of radiopacifiedtricalcium silicate cement, Biodentine and MTA Angelus. Dental materials.2013;29: 580 – 593.

This work is licensed under a Creative Commons Attribution 4.0 International License.









