Synthesis and Antioxidant Ability of Some 4-(((4-(5-(Aryl)-1,3,4-Oxadiazol-2-Yl)Benzyl)Oxy)Methyl)-2,6-Dimethoxyphenol
Raied Mustafa Shakir
Department of Chemistry, Ibn Al-haitham, University of Baghdad, Baghdad 61023, Iraq.
Corresponding Author E-mail: raiedalsayab@yahoo.co.uk
DOI : http://dx.doi.org/10.13005/ojc/320533
A series of new 4-(((4-(5-(Aryl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxy phenol (6a-i) were synthesized from cyclization of 4-(((4-hydroxy-3,5-dimethoxy benzyl)oxy)methyl)benzohydrazide with substituted carboxylic acid in the presences of phosphorusoxy chloride.The resulting compounds were characterized by IR, 1H-NMR, 13C-NMR, and HRMS data. 2,2-Diphenyl-1-picrylhydrazide (DPPH) and ferric reducing antioxidant power (FRAP) assays were used to screen their antioxidant properties. Compounds 6i and 6h exhibited significant antioxidant ability in both assay. Furthermore, type of substituent and their position of the aryl attached 1,3,4-oxadiazole ring at position five are play an important roles in enhancing or declining the antioxidant properties.
KEYWORDS:2,6-dimethoxyphenol,1,3,4-oxadiazole; antioxidant; DPPH; FRAP
Download this article as:| Copy the following to cite this article: Shakir R. M. Synthesis and Antioxidant Ability of Some 4-(((4-(5-(Aryl)-1,3,4-Oxadiazol-2-Yl)Benzyl)Oxy)Methyl)-2,6-Dimethoxyphenol. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Shakir R. M. Synthesis and Antioxidant Ability of Some 4-(((4-(5-(Aryl)-1,3,4-Oxadiazol-2-Yl)Benzyl)Oxy)Methyl)-2,6-Dimethoxyphenol. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21329 |
Introduction
the free radicals are qualified to cause grave damage to biomolecules, including proteins, lipids, DNA, and carbohydrates.1 This damage lead to causes many diseases such as inflammatory2 and cancers disease 3, degenerative disease4 and Chronic diseases. This actuality make the free radical scavenging compounds are interesting for their ability to terminate or reduce the oxidation and inhibiter the free radical effect. On the other hand, the anti-inflammatory, digestive, anti-necrotic, hepatopr-otective, and neuroprotective drugs own an antioxidant ability5 as well, the antioxidants have been reported it shows potential adverse health effects.6
Ordinarily, antioxidants compounds donate protons to become more stable free radicals. This stability increases with the extent of delocalization and enhances the antioxidant ability7,8 . Furthermore, the antioxidants usually own common structural features such as multiple phenolic hydroxyl groups like flavonoids 9 or have full conjugation π system like carotenoids10. Moreover, exhibited substituted groups might influence on the scavenging ability. This indicates the existence of a close relationship between the chemical structure and the ability to scavenge free radicals.
The 1,3,4-oxadiazoles derivatives are known with their wide spectrum of biological activities11-14 besides the antioxidant ability7,15,16. As for 2, 6-dimethoxyphenol derivetives, there has been exhibited interest during the last years as antioxidant materials17,18 . In this work we presented the synthesis of new 4-(((4-(5-(Aryl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxy phenol (6a-i) as promising antioxidant material.
Material and Method
Chemistry
The IR spectra were obtained with a Perkin Elmer 400 Fourier Transform Infrared (FTIR) Spectrometer. 1H and 13C-NMR spectra were recorded at Joel Lambda spectrometers at 400 MHz) UM, Malaysia). CDCl3 and DMSO-d6 were used as solvents with TMS as the internal standard. Agilent 5975 and a Finnigan TSQ7000 were used to determine the EI/Ms and HREIMs (NUS, Singapore) respectively. FRAP and DPPH.were record by UV spectroscopy, a Power Wave X340 (BIO-TEK Instruments, Inc., Winooski, VT, USA). Melting points were measured with OMEGA MPS10 melting point apparatus in open-end capillary tubes. Flash Column chromatographic purification was carried out using silica gel 60 (230–400 mesh, E. Merck) was employed. Reagents and solvents were purchased from commercial suppliers without further purification.
3,5-dimethyl-4-((trimethylsilyl)oxy)benzyl alcohol 1
This compound was synthesized as reported by Ali, K.F.19. the crude product was purify by column chromatography using (6-1) hexane ethyl acetate as eluent to give pale yellow oil Yield 83 %, Bp 312-314 ºC at 760 mmHg, d=1.125 at 25 ºC [314-317 ºC lit. 19]. IR (liquid film) vmax 3332(OH), 3060(CHAr), 2962, 2877 (CHaliphatic), 1595(C=C), 1201 (Ar-O-C), 862(Si-CH3) cm-1, 1H-NMR(400MHz, CDCl3): δ 0.23(9H, s, Si-(CH3)3), 2.95 (1H, bs, OH), 3.82 (6H, s, 2×OCH3), 4.50(2H, s, OCH2), 6.70 (2H, s, H-3). 13C-NMR (100 MHz, CDCl3): δ -0.05 (3C, Si-(CH3)3, 55.46(2C, OCH3), 61.87(1C, CH2OH), 106.82(2C, CH), 127.94(1C), 135.70(1C), 151.95(1C), HREIMs m/z = 256.1127 [M˙+] (calc. for C12H20O4Si, 256.1131).
Synthesis of methyl 4-(((3,5-dimethoxy-4-((trimethylsilyl)oxy)benzyl)oxy)methyl) benzoate 3
Methyl 4-(bromomethyl)benzoate (4.58g, 20 mmol) was added in small portions to a stirring solution of 3,5-dimethyl-4-((trimethylsilyl)oxy)benzyl alcohol (4.84 g,20 mmol) in 25 mL dry pyridine within 45 minutes. After complete the addition the mixture was refluxed for 12 hours. Upon cooling the mixture poured in to 100 mL crashed ice and acidified with 5% of hydrochloric acid. The product was extracted with ethyl acetate 25 mL×3 and washed with water, then dried under magnesium sulfate. After evaporated the solvent, the crude material was purified by column chromatography using hexane-ethyl acetate (8:1) as eluent to obtain pale yellow oil which is solidify after cooling to 5 ºC to obtain white solid. Yield 67%, Mp 8-10 ºC, IR (liquid film) vmax 3030 (CHAr), 2966, 2890 (CHaliphatic), 1728(C=O),1595(C=C), 1195 (Ar-O-C), 867(Si-CH3) cm-1. 1H-NMR (400MHz, CDCl3): δ 0.18 (9H, s, Si-(CH3)3), 3.81 (6H, s, 2×OCH3), 3.85(3H, s, OCH3), 4.32 (2H, s, OCH2), 4.37 (2H, s, OCH2), 6.64 (s, 2H, H3), 7.43 ( 2H, d, J 8.1, H8),7.51( 2H, d, J 8.2, H9). 13C-NMR (100 MHz, CDCl3): δ -0.053 (3C, Si-(CH3) 3), 51.2(1C,OCH3), 57.02(2C, OCH3), 70.77(1C, CH2OCH2), 71.19(1C, CH2OCH2), 107.70 (2C, CH), 127.89 (1C), 128.55(2C, CH), 131.87 (2C, CH), 135.43 (1C), 138.44 (1C), 140.2(1C) 152.18(2C),166.5 (1C, C=O). HREIMs m/z = 404.1651 [M˙+] (calc. for C21H28O6Si, 404.1655).
4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzoic acid 4
Stirred mixture of methyl4-(((3,5-dimethoxy-4-((trimethylsilyl)oxy)benzyl)oxy)methyl) benzoate(8.08 g , 20mmol) in 10 mL methanol and 20 mL of 50% acetic acid was heated under reflex overnight. The solvent was removed under reduced pressure and then 25 mL of 10% sodium hydrogen carbonate was added and heated for 30 minute . After cooling the mixture was extracted from ethyl acetate . The organic layer was ignored and the aqueous layer was acidified .by 5% hydrochloric acid. The precipitated collected by filtration and washed with water. Recrystallized from ethanol to obtain white microcrystals. Yield 89 %, Mp 153-155 C [ lit. 152-154 ̊C 19], , IR (KBr) vmax 3354 (OH), 3046 (CHAr), 2972, 2893 (CHaliphatic), 1676 (C=O) 1585 (C=C), 1201 (Ar-O-C) cm-1. 1H-NMR (400MHz, DMSO-d6): 3.87 (6H, s, 2×OCH3), 4.31 (2H, s, OCH2), 4.38 (2H, s, OCH2), 6.66 (2H, s, H3), 7.65 ( 2H, d, J 8.2, H8), 8.12 ( 2H, d, J 8.1, H9). 9.15 (1H, bs, OH), 13C-NMR (100 MHz, DMSO-d6): 56.11(2C, OCH3), 71.22(1C, CH2OCH2), 72.77 (1C, CH2OCH2), 109.05 (2C, CH), 128.84 (2C, CH), 129.37 (1C), 131.21 (2C, CH), 134.23 (1C), 139.65 (1C), 142.13 (1C) 152.01 (2C), 169.58 (1C, C=O) HREIMs m/z = 318.1100 [M˙+] (calc. for C17H18O6, 318.1103)
4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzohydrazide 5
This compound was synthesized according to the procedure described by K. F. Ali 19.The crude was recrystallized from ethanol to give white solid. Yield 87%, Mp 94-96 ̊ C [ lit. 92-94 ̊C 19] , IR (KBr) vmax 3418 (OH phenol), 3326, 3209 (NH, NH2), 3061 (CHAr), 2976 -2875 (CHaliphatic), 1664 (C=O), 1592 (C=C), 1197(Ar-O-C), cm-1. 1H-NMR (400MHz, DMSO-d6): 3.82 (6H, s, 2× OCH3), 4.16 (2H, s, OCH2), 4.40 (2H, s, OCH2), 4.73 (2H, bs, NH2), 6.62 (2H, s, H3), 7.69 ( 2H, d, J 8.2, H8), 8.24( 2H, d, J 7.94, H9), 8.63 (1H, bs, CONH), 9.44 (1H, bs, OH), 13C-NMR (100 MHz, DMSO-d6): 56.6 (2C, OCH3), 71.09 (1C, CH2OCH2), 72.82 (1C, CH2OCH2), 108.87(2C, CH), 128.74(1C), 129.22 (2C,CH), 130.01(2C, CH), 132.92(1C), 138.71(1C), 141.48(1C) 151.92 (2C), 166.05(C=O). HREIMs m/z = 332.1367 [M˙+] (calc. for C17H20N2O5, 332.1372)
General Synthesis of 4-(((4-(5-(Aryl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxyphenol 6a-6i
A mixture of 4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzohydrazide (0.33 g, 0.1 mmol) and substituted carboxylic acid (0.1 mmol) in 50 mL round flask, 5 mL of phosphorusoxy chloride was added in a few portions at room temperature. The mixture was stirred and refluxed for four hours in a water bath at 85-95 °C. After cooling, the mixture was poured into 100 mL crushed ice and stirred for 15 minutes. Ammonium solution 10 % was added in few portions until the pH adjusted to 7-8. The precipitate was filtered, washed with water and dried. The crude product was purified either from column chromatography or recrystallized from suitable solvent.
4-(((4-(5-(4-methylphenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxyphenol 6a
Crude product was recrystallized by methanol to afford white precipitate .Yield 69% ,Mp 174-176 ˚C. IR (KBr) vmax 3559 (OH), 3071 (CHAr), 2953, 2872 (CHaliphatic),1608 (C=N), 1595 (C=C). 1249 (C-N), 1101(C-O-C) cm-1. 1H-NMR(400MHz, CDCl3), 2.32 (s, 3H, p-CH3-ph), 3.79 (6H, s, 2× OCH3), 4.11 (2H, s, OCH2), 4.37 (2H, s, OCH2), 6.62 (2H, s, H3), 7.32 (2H, d, J 8.26, H15, H17), 7.69 ( 2H, d, J 8.2, H8), 8.03 (d, 2H, J 8.26, H14, H18); 8.24 (2H, d, J 7.94, H9), .13C NMR (CDCl3, 100 MHz, ppm): 21.73 (p-CH3ph), 56.5 (2C, OCH3), 71.11 (1C, CH2OCH2), 72.79 (1C, CH2OCH2), 108.83 (2C, CH), 115.41(1C), 121.56(1C), 126.66(2C, CH), 128.71(1C), 129.17 (2C, CH), 129.69(2C, CH), 129.91(2C, CH), 138.82 (1C), 141.39 (1C) 142.03 (1C) 151.85 (2C), 164.13 & 165.23 (2C, C=N). HREIMs m/z = 432.1681 [M˙+] (calc. for C25H24N2O5, 432.1685).
4-(((4-(5-(4-methoxyphenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxyphenol 6b
The product was recrystallized by ethanol to give white needle crystals, Yield 61% Mp 153-155 ˚C. IR (KBr) vmax 3570 (OH), 3067 (CHAr), 2983, 2854 (CHaliphatic),1611 (C=N), 1592 (C=C). 1251(C-N), 1091(C-O-C) cm-1. 1H-NMR(400MHz, CDCl3), 3.79 (6H, s, 2× OCH3), 3.88 (3H, s, OCH3), 4.11 (2H, s, OCH2), 4.37 (2H, s, OCH2), 6.62 (2H, s, H3), 7.05 (2H, d, J 9.06, H15, H17), 7.99 ( 2H, d, J 8.02, H-8), 8.11 (2H, d, J 8.50, H14, H18); 8.20( 2H, d, J 8.0, H9). 13C NMR(CDCl3, 100 MHz, ppm) 55.47 (OCH3), 56.5 (2C, OCH3), 71.11 (1C, CH2OCH2), 72.79 (1C, CH2OCH2), 108.83(2C, CH), 114.41 (2C, CH), 115.37 (1C), 116.72 (1C), 127.97 (2C,.CH), 128.71(2C, CH), 129.17 (1C), 129.69 (2C, CH), 138.92 (1C), 142.09 (1C), 150.23 (2C), 161.85 (1C), 163.88 & 164.77 (2C, C=N). HREIMs m/z = 448.1629 [M˙+] (calc. for C25H24N2O6, 448.1634).
4-(((4-(5-(4-ethoxyphenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxyphenol 6c
The solid product recrystallized from acetonitrile to obtain white amorphous. Yield 63% Mp 144-146 ˚C. IR (KBr) vmax 3564 (OH), 3081 (CHAr), 2960, 2857 (CHaliphatic),1607 (C=N), 1595 (C=C). 1244 (C-N), 1101(C-O-C) cm-1. . 1H-NMR(400MHz, CDCl3), 1.45 (3H, t, J 7.42, OCH2CH3), 3.69 (6H, s, 2× OCH3), 4.14 (2H, q, J 7.8, OCH2), 4.28 (2H, s, OCH2), 4.37 (2H, s, OCH2), 6.62 (2H, s, H-3), 7.21(2H, d, J 8.8, H15, H17), 7.99 (d, 2H, J 8.02, H8), 8.02(2H, d, J 8.04, H14, H18); 8.20(2H, d, J 8.0, H9). 13C NMR (CDCl3, 100 MHz, ppm), 14.82 (1C, CH3), 55.25 (2C, OCH3), 63.84 (OCH2CH3), 70.97(1C, CH2OCH2), 71.77 (1C, CH2OCH2), 108.08 (2C, CH), 113.65 (1C), 114.09 (2C,CH), 114.78 (1C), 115.57 (1C), 127.83 (2C,CH), 128.47 (2C,CH), 129.04 (1C), 129.60 (2C, CH), 137.79 (1C), 141.50 (1C), 153.01 (2C), 158.79 (1C), 163.86 & 165.03 (2C, C=N). HREIMs m/z = 462.1788 [M˙+] (calc. for C26H26N2O6, 462.1791).
4-(((4-(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxyphenol 6d
The product was recrystallized from acetonitrile to afford pale yellow precipitate. Yield 76%, Mp 160-162 ºC. IR (KBr) vmax 3565 (OH), 3075 (CHAr), 2967, 2883 (CHaliphatic), 1610 (C=N), 1595 (C=C), 1240 (C-N), 1106 (C-O-C) cm-1. 1H-NMR(400MHz, CDCl3), 3.87 (6H, s, 2×OCH3), 4.31 (2H, s, OCH2), 4.38 (2H, s, OCH2), 6.66 (2H, s, H3), 7.49 (2H, d, J 8.24, H15, H 17), 7.65 ( 2H, d, J 8.2, H8), 8.05 (2H, d, J 8.23, H14, H18), 8.12 ( 2H, d, J 8.1, H9). 9.19 (1H, bs, OH), 13C-NMR (100 MHz, CDCl3): 56.11(2C, OCH3), 71.12 (1C, CH2OCH2), 72.75 (1C, CH2OCH2), 109.25 (2C, CH), 122.74 (1C), 127.81 (2C, CH), 128.03 (2C, CH), 128.97 (2C, CH), 130.11 (2C,CH), 131.21 (1C), 136.23 (1C), 137,28(1C),139.65 (1C), 141.13(1C), 152.01 (2C),164.68 & 164.89 (2C, C=N). HREIMs m/z = 452.1135 [M˙+] (calc. for C24H21ClN2O5, 452.1139).
4-(((4-(5-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxy phenol 6e
The solid crude was purified by column chromatography using (6:1) hexane: ethyl acetate as eluent and was then recrystallized from ethyl acetate to give white crystal. Yield 62%, Mp 208-210˚C. IR (KBr) vmax 3617 (br, OH phenol),), 3060 (CHAr), 2986 -2865 (CHaliphatic), 1609 (C=N), 1588 (C=C), 1198 (Ar-O-C), cm-1. 1H-NMR (400MHz, DMSO-d6): 3.84 (6H, s, 2× OCH3), 4.19 (2H, s, OCH2), 4.42 (2H, s, OCH2), 6.62 (2H, s, H3), 7.01 (2H, d, J 8.66, H14, H18), 7.71 ( 2H, d, J 8.2, H8), 8.01(2H, d, J.8.8, H15, H17); 8.21( 2H, d, J 7.94, H9), 9.23 (1H, bs, OH), 9.48 (1H, bs, OH), 13C-NMR (100 MHz, DMSO-d6): 56.6 (2C, OCH3), 71.09 (1C, CH2OCH2), 72.82 (1C, CH2OCH2), 108.87(2C, CH), 115.58 (1C), 116.40(2C, CH), 128.97 (2C, CH). 129.22 (2C, CH), 130.01 (2C, CH), 132.92 (1C), 138.71 (1C), 141.48 (1C), 142.09 (1C), 159.83 (C10), 151.90 (2C), 164.53 & 165.15(2C, C=N). HREIMs m/z = 434.1474 [M˙+] (calc. for C24H22N2O6, 434.1478
4-(((4-(5-(3,4-dichlorophenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxyphenol 6f
The crude solid was recrystallized from acetonitrile to give white solid. Yield 81%, Mp 214-216˚C, IR (KBr) vmax 3422 (OH phenol), 3061 (CHAr), 2970 -2845 (CHaliphatic), 1614 (C=N), 1590 (C=C), 1205 (Ar-O-C), cm-1. 1H-NMR (400MHz, CDCl3): 3.82 (6H, s, 2× OCH3), 4.16 (2H, s, OCH2), 4.40 (2H, s, OCH2), 6.62 (2H, s, H3), 7.57 (1H, d, J 8.52, H17), 7.69 ( 2H, d, J 8.2, H8), 7.91 (1H, dd, J 7.8, 1.95, H18), 8.19 (1H, d, J 2.2, H14) 8.24 (2H, d, J 7.94, H9), 9.49 (1H, bs, OH), 13C-NMR (100 MHz, CDCl3): 56.6 (2C, OCH3), 71.09 (1C, CH2OCH2), 72.82 (1C, CH2OCH2), 108.67(2C, CH), 124.01 (1C), 126.15 (1C), 128.33 (1C), 128.70(1C), 129.51 (2C,CH), 130.34 (2C, CH), 132.08 (1C), 132.75(1C), 134.17 (1C), 135.79 (1C), 138.76 (1C), 141.52 (1C) 152.02 (2C), 162.5 & 165.88 (2C, C=N). HREIMs m/z = 486.0745 [M˙+] (calc. for C24H20Cl2N2O5, 486.0749).
4-(((4-(5-(3,5-dichlorophenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxy phenol 6g
The product was recrystallized by acetonitrile to give white needle crystals, Yield 74% Mp 188-190 ˚C. IR (KBr) vmax 3559 (OH), 3080 (CHAr), 2988, 2864 (CHaliphatic), 1610 (C=N), 1595 (C=C). 1249 (C-N), 1098 (C-O-C) cm-1. 1H-NMR(400MHz, CDCl3), 3.75 (6H, s, 2× OCH3), 3.87 (3H, s, OCH3), 4.14 (2H, s, OCH2), 4.35 (2H, s, OCH2), 6.59 (2H, s, H3), 6.62 (2H, s, H3), 7.52 (1H, t, J 1.44, H16), 7.64 (2H, d, J 8.22, H8), 8.04 (2H, t, J 1.70, H14, H18), 8.26 (2H, d, J 7.96, H9), 9.53 (1H, bs, OH). 1H NMR (CDCl3, 400 MHz, ppm):56.6 (2C, OCH3), 71.09 (1C, CH2OCH2), 72.82 (1C, CH2OCH2), 108.87(2C, CH), 125.02 (2C, CH), 126.87 (1C), 129.22 (2C, CH), 130.01(2C, CH), 131.24 (1C), 132.92 (1C), 135.92 (2C), 138.71(1C), 141.48(1C), 141.98 (1C), 151.92 (2C), 162.43 & 165.86 (2C, C=N). HREIMs m/z = 486.0744 [M˙+] (calc. for C24H20Cl2N2O5, 486.0749)
4-(((4-(5-(3,5-di-tertbutyl-4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxyphenol 6h
The crude material was purified by column chromatography using (hexane-ethyl acetate) 6-1 as elute to give a white solid. Yield 56%; m.p.181-183 °C; IR (KBr) vmax 3538 (OH), 3079 (CHAr ), 2983, 2853 (CHaliphatic), 1615 (C=N), 1592 (C=C). 1231 (C-N), 1105 (C-O-C) cm-1. 1H-NMR (400MHz, CDCl3): 3.87(6H, s, 2 × OCH3), 3.89 (6H, s, 2 × OCH3), 4.31 (2H, s, OCH2), 4.38 (2H, s, OCH2), 6.62 (2H, s, H14, H18), 6.66 (2H, s, H3), 7.65 ( 2H, d, J 8.2, H8), 8.12( 2H, d, J 8.1, H9). 9.15 (1H, bs, OH), 9.11 (1H, bs, OH), 13C-NMR (100 MHz, DMSO-d6): 56.6 (4C, OCH3), 71.09 (1C, CH2OCH2), 72.82 (1C, CH2OCH2), 105.44(2C, CH), 108.87 (2C, CH), 119.07 (1C), 129.22 (2C, CH), 130.01 (2C, CH), 132.92 (1C),137.71 (2C), 138.71 (1C), 140.71 (1C), 141.48 (1C), 150.11 (1C), 151.92 (2C), 164.62 & 164.94 (2C, C=N). HREIMs m/z = 494.1687 [M˙+] (calc. for C26H26N2O8, 494.1689).
4-(((4-(5-(3,5-dimethoxy-4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxyphenol 6i
The crude solid was purified by column chromatography using (hexane-ethyl acetate) 9-1 as elute to afford white amorphous. Yield 60 % Mp 204-206˚C. IR (KBr) vmax 3478 (OH), 3072 (CHAr), 2987, 2854 (CHaliphatic), 1608 (C=N), 1595 (C=C). 1242 (C-N), 1094(C-O-C) cm-1. 1H-NMR (400MHz, DMSO-d6): 1.53(18H, s, 2× C(CH3)3), 3.82 (6H, s, 2× OCH3), 4.16 (2H, s, OCH2), 4.40 (2H, s, OCH2), 5.66(1H, s, OH),6.62 (2H, s, H3), 7.69 ( 2H, d, J 8.2, H8), 7.92 (2H, s, H13), 8.21(2H, d, J 7.96, H9), 9.48 (1H, bs, OH), 13C-NMR (100 MHz, DMSO-d6): 30.23 (6C, 2×C(CH3)3), 34.56(2C, 2×C(CH3)3), 56.6 (2C, OCH3), 71.09 (1C, CH2OCH2), 72.82 (1C, CH2OCH2), 108.87 (2C, CH), 115.12 (1C), 124.43 (1C), 128.74 (1C), 129.22 (2C, CH), 130.01(2C, CH), 132.92 (1C), 136.87 (2C) 138.71 (1C), 141.48 (1C) 151.92 (2C), 157.33 (1C), 163.37 & 165.66 (2C, C=N). HREIMs m/z = 546.2725 [M˙+] (calc. for C32H38N2O6, 546.2730).
Antioxidant
DPPH assay
The assay was achieved as reported by Gerhauser et al., 20. Five microliters of the sample in ethanol was added into 195 μL of 100 μM DPPH reagent in ethanol (96%) and mixed in a 96-well plate. The intensity of the colour was measured for 3 h at a period of 20 min. at 515 nm. Ascorbic acid , BHT and 2,6-dimethoxyphenol were used as reference.
FRAP assay
The FRAP assay was achieved as reported by Benzie and Strain 21 method. The FRAP reagent was prepared by combining 300 mM acetate buffer and 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) solution in 40 mM HCl and 20 mM FeCl3·6H2O, in a ratio of 10:1:1. The FRAP reagent was incubated at 37 °C prior to use. Ten microliters of the sample was reconstituted in the carrier (solvent or ultrapure water) and mixed with 300 μL of FRAP reagent. The mixture was incubated at 37 °C for 4 min. in a microplate reader. The absorbance of the complex was 593 nm. The FRAP value was calculated using the following equation22 :
FRAP = [(0–4 min ∆A593 nm of test sample)/(0–4 min. ∆A593 nm of standard)]
× [standard] (µM) × Y × 1000
Where; Y is absorbance of the spectrophotometer
Results and Discussion
Chemistry
The 3,5-dimethyl-4-((trimethylsilyl)oxy)benzyl alcohol was synthesized according to the procedure described by Ali, K.F.19. the resulting compound was reacted with Methyl 4-(bromomethyl)benzoate in dry pyridine to preform 4-(((3,5-dimethoxy-4-((trimethylsilyl) oxy)benzyl)oxy)methyl) benzoate 3. The hydrolysis of this compound afforded compound 4. The carboxylic was converted to their corresponding hydrazide 5. Finally the hydrazide was reacted with ni ne substituted benzoic acid in the presence of phosphorusoxy chloride as dehydration agent to formed the 1,3,4-oxadiazole ring (6a-i) as depicted in scheme 1
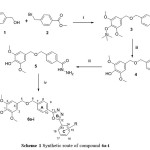 |
Scheme 1: Synthetic route of compound 6a-i |
The newly 1,3,4-oxadiazole were characterized from their IR, 1H NMR, 13C NMR and HRMs. The IR spectra showed disappearing the signal of carbonyl group acid As well the NH, NH2 and the OH for the hydrazide and carboxylic acid The new interesting signal of C=N of the oxadiazole ring was located at 1607-1615 cm-1. The aryl group and some selected properties of these compounds were tabulated in Table 1
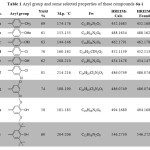 |
Table 1: Aryl group and some selected properties of these compounds 6a-i. |
The 1H NMR spectra of 6a-i displayed disappearing signals of the NH, NH2 and OH group of the hydrazide and the carboxylic acid. The protons of the benzyloxymethyl-2,6-dimethoxyphenol group were appeared at the expected rang. Furthermore, the 1HNMR spectra exhibited new interesting peaks for the aryl group of 5-aryl-1,3,4-oxadiazole besides their substituted group. For example, the methyl group of compound 6a appeared as signal peak at 2.32 ppm with integration of three protons also the ethoxy group of compound 6c exhibited as two signal the first one appeared as triplet peak at 1.45 with coupling constant(J) equal 7.42 Hz and the second one appeared as quartet peak at 4.14 with J= 7.8 Hz. The 13C NMR spectra of these compounds exhibited disappearing the carbonyl group of the starting material as well new two interesting peaks at 162.43-164.68 ppm and 164.77-165.88 ppm indicated the two carbons, C11 and C12 of C=N in oxadiazole ring. All expected carbons were appeared at their expected area. The EIMS spectra exhibited the molecular ion M•+ for all newly synthesized compounds besides the value of base peak (100%) . The HREIMs value was confirmed the accurate mass and the molecular formula as depicted in Table 1.
Antioxidant activity
The antioxidant activities of the synthesized compounds 6a-i were tested by DPPH and FRAP assays. Differences between the structures of these compounds occurred in ring C which has different substituent at different positions, whereas rings A and B possess the same structure. Various antioxidant abilities were displayed in both assays according to the type of substituent and their position, which are play an important roles in enhancing or declining the antioxidant ability. Furthermore, the inductive effects and the mesomeric effect beside the position directly affected antioxidant ability7. Compound 6i with 3,5-di-tert-butyl phenol attached the oxadiazole at position five showed the significant antioxidant ability in both assays. The DPPH percentage of inhibition (92.03±0.121) and lowest IC50 (20.19±14) and frap value 968.1 (slightly higher than ascorbic acid).Compound 6h displayed DPPH inhibition % slightly less than ascorbic acid and slightly higher IC50. Also the 6e exhibited good free radical scavenging ability These results are consistent with the concept that the hydroxyl group also the sterical hindrance enhances the antioxidant ability.23-26 The DPPH inhibition %, FRAP values and the substituent followed the following sequence; 3,5-di-tert-butyl-4-OH > 3,5-di-OMe-4-OH > 4-OH > 4-Me > 4-OMe ≈ 4-OEt > 4-Cl > 3,4-di-Cl ≈ 3,5-di-Cl, as depicted in Table 2. This sequence showed that the electron-releasing group, which exerts mesomeric and inductive effects beside their position, increases the antioxidant ability, while the inductive-withdrawing group decreases antioxidant ability.
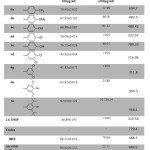 |
Table 2: Antioxidant activity of the synthesized compounds 6a-i
|
Conclusions
A series of new 4-(((4-(5-(Aryl)-1,3,4-oxadiazol-2-yl)benzyl)oxy)methyl)-2,6-dimethoxy phenol (6a-i) were successfully synthesized and characterized. The antioxidant activity for these compounds were tested by DPPH and FRAP. The antioxidant results showed that the type of substituent and their position of the aryl attached 1,3,4-oxadiazole ring at position five are play an important roles in enhancing or declining the antioxidant properties.
Acknowledgements
The author would like to thank the University of Malaya for running the NMR and to NUS, (Singapore) for running the EIMS and HREIMS. We are also grateful to Dr Mohammed Farouq Halabi, for his great help with screening antioxidant assys. As well, we would like to thank the university of Baghdad for supporting this study and provide the grant for this study
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
References
- Shacter, E. Drug Metabolism Reviews 2000, 32, 307.
CrossRef - Chatterjee, R.; Bandyopadhyay, U.; Mazumdar, A.; Banerjee, R. K. Biochemical Pharmacology 1996, 52, 1169.
CrossRef - Sangeetha, P.; Das, U. N.; Koratkar, R.; Suryaprabha, P. Free Radical Biology and Medicine 1990, 8, 15.
CrossRef - Kar, S.; Subbaram, S.; Carrico, P. M.; Melendez, J. A. Respir Physiol Neurobiol. 2010, 174, 299.
CrossRef - Lin CC, H. P. Phytotherapy Research 2000, 14, 489.
CrossRef - Chattopadhyay, M. K. Current Science 2003, 85, 121.
- Shakir, R. M.; Ariffin, A.; Abdulla, M. A. molecules 2014, 19, 3436.
- Hung, C.-Y.; Yen, G.-C. J. Agric. Food Chem. 2002, 50, 2993
CrossRef - Jeong , J.-M.; Kang, S.-K.; Lee, I.-H.; Lee, J.-Y.; Jung, H.; Choi, C.-H. J Pharm Pharmaceut Sci 2007, 10, 537.
CrossRef - Edge, R.; McGarvey, D. J.; Truscott, T. G. J Photoch Photobio B 1997, 41, 189.
CrossRef - Dhumal, S. T.; Deshmukh, A. R.; Bhosle, M. R.; Khedkar, V. M.; Nawale, L. U.; Sarkar, D.; Mane, R. A. Bioorganic & Medicinal Chemistry Letters 2016, 26, 3646.
CrossRef - Tyagi, M. Orient J Chem 2014, 30, 713.
- Sreeramulu J, A. P. Orient J Chem 2014, 30, 651.
- Mochona, B.; Qi, X.; Euynni, S.; Sikazwi, D.; Mateeva, N.; Soliman, K. F. Bioorganic & Medicinal Chemistry Letters 2016, 26, 2847.
CrossRef - Sumangala Vittal, B. P., Punith Bansal, Chidananda Nandagokula, ArulMoli, Tangavelu and Shalini Shenoy Der Pharma Chemica 2011, 3, 138.
- Ma, L.; Xiao, Y.; Li, C.; Xie, Z.-L.; Li, D.-D.; Wang, Y.-T.; Ma, H.-T.; Zhu, H.-L.; Wang, M.-H.; Ye, Y.-H. Bioorganic & Medicinal Chemistry 2013, 21, 6763.-6770
- Huihui Ti; Qing Li; Ruifen Zhang; Mingwei Zhang; Yuanyuan Deng; Zhencheng Wei; Jianwei Chi; Yan Zhang Food Chemistry 2014, 159 166.
- Fausta Natella, M. N., Maurizio Di Felice, and Cristina Scaccini*; Free Radical Research Group, N. I. o. N., Roma, Italy J. Agric. Food Chem. 1999, 47, 1453.
- Ali, K. F. Orient J Chem., 2015, 31, 239.
- Gerhäuser, C.; Klimo, K.; Heiss, E.; Neumann, I.; Gamal-Eldeen, A.; Knauft, J.; Liu, G.-Y.; Sitthimonchai, S.; Frank, N. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2003, 523-524, 163.
CrossRef - Benzie, I. F.; Strain, J. J. Anal. Biochem. 1996, 239, 70.
CrossRef - Rustaiyan, A., Javidnia, K., Farjam, M H., Aboee-Mehrizi, F., Ezzatzadeh, E. J Med Plants Res. 2011, 5, 4251.
- Shahidi, F. Natural Antioxidants: Chemistry, Health Effects, and Applications; ACOS,: USA 1997.
- Simić, A., Manojlović, D., Šegan, D., Todorović, M. Molecules 2007, 12, 2327.
CrossRef - Moalin, M.; Strijdonck, G. P. F.; Beckers, M.; Hagemen, G. J.; Borm, P. J.; Bast, A.; Haenen, G. R. M. M. Molecules 2011, 16, 9636.
CrossRef - Torres, R., Urbina, F., Morales, C., Modak, B., Monache, F D. J. Chil. Chem. Soc. 2003, 48, 0717.

This work is licensed under a Creative Commons Attribution 4.0 International License.









