Sorption Isotherm Model of Zinc (II) onto Thermally Treated Rice Husk
Ahmad Farhan Ahmad Tarmizi1, Keat Khim Ong2*, Wan Md Zin Wan Yunus1, Anwar Fitrianto3, Mohd Lip Jabit4, Abdul Ghapor Hussin1 and Chin Chuang Teoh5
1Department of Defence Science, Faculty of Defence Science and Technology, Universiti Pertahanan Nasional Malaysia, Malaysia.
2Department of Chemistry and Biology, Centre for Defence Foundation Studies, Universiti Pertahanan Nasional Malaysia, Malaysia.
3Department of Mathematics, Faculty of Science, Universiti Putra Malaysia, Malaysia.
4Technical Services Centre, Malaysian Agricultural Research and Development Institute (MARDI) Headquarter, G. P.O. Box 12301, 50774 Kuala Lumpur, Malaysia.
5Engineering Research Centre, Malaysian Agricultural Research and Development Institute, (MARDI) Headquarter, G. P.O. Box 12301, 50774 Kuala Lumpur, Malaysia.
*Corresponding Author E-mail: ongkhim@upnm.edu.my
DOI : http://dx.doi.org/10.13005/ojc/320555
Various modifications were used to convert waste materials into heavy metal sorbents. In this study, simple thermal treatment was applied on rice husk and its sorption potential to remove Zn (II) ions from aqueous solution was investigated. Equilibrium sorption data were evaluated with two commonly used isotherms i.e. Langmuir and Freundlich isotherms. Coefficient of determination (R2) of the models were used to determine the best fitted isotherm. The correlation coefficients were 0.9884 and 0.8496 for Langmuir and Freundlich isotherms, respectively, showing that the sorption was better described by Langmuir isotherm. In addition, the sorption process is found to be favourable as indicated by the separation factor (RL) value between 0 and 1. The potential of thermally treated rice husk as a sorbent for the removal of Zn (II) ions from aqueous solution is recognised.
KEYWORDS:Isotherm Model; Thermally Treated Rice Husk; Zn (II) Ions
Download this article as:| Copy the following to cite this article: Tarmizi A. F. A, Ong K. K, Yunus W. M. Z. W, Fitrianto A, Jabit M. L, Hussin A. G, Teoh C. C. Sorption Isotherm Model of Zinc (II) onto Thermally Treated Rice Husk. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Tarmizi A. F. A, Ong K. K, Yunus W. M. Z. W, Fitrianto A, Jabit M. L, Hussin A. G, Teoh C. C. Sorption Isotherm Model of Zinc (II) onto Thermally Treated Rice Husk. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21435 |
Introduction
Heavy metals are toxic and hazardous pollutants in water caused by due to their non-biodegradable and persistent behaviours in the environment1. Zinc is one of the heavy metals discharged from industrial effluents. Its harmful health effects including “metal-fume fever”, nausea, diarrhoea, depression, lethargy, seizures, and ataxia2. Consequently, the maximum acceptable concentration of Zn (II) in drinking water permitted by World Health Organization (WHO) is 0.05 mg/L3.
Different chemical and physical methods such as precipitations, ion exchange, filtration, and solvent extraction have been applied to remove heavy metals from wastewater. However, these methods have some drawbacks for examples high operational cost, low removal efficiency at low concentration, and producing toxic sludge that needs further treatment4. Nevertheless, adsorption is an alternative approach to treat toxic heavy metals since this method is widely used as it is cost effective process5. Various waste materials have been used as adsorbents for examples rapeseed waste6, coffee husks7, corn cob8, alum sludge waste9, and rice husk10.
Understand adsorption mechanism by adsorption isotherm study is important as it can shows the distribution of adsorbate molecules between the liquid phase onto the solid phase. Langmuir and Freundlich models are the common isotherm models used to correlate the adsorption equilibrium of heavy metals onto adsorbents12. Paduraru et al.6 and Oliveira et al.7 have shown that equilibrium data fitted well with Langmuir isotherm model compared to Freundlich in their investigations on adsorption of Zn (II) ions onto rapeseed and coffee husks, respectively.
According to Abdolali et al.11, pre-treatment of adsorbents can improve physical and chemical properties of adsorbents, which can enhance the adsorption capacity and prevent organic leaching. Hence, in this work, a simple treatment was applied on rice husk to produce thermally treated rice husk for sorption of Zn (II) ions from aqueous solution. Equilibrium isotherm of the sorption was investigated.
Materials and Methods
Chemicals
Sodium hydroxide pellets, 1.0 M hydrochloric acid and 1000 mg/L of zinc nitrate standard solutions were of analytical grade and obtained from Merck Company (Germany). Ultrapure water (resistivity of 18.2 MΩ.cm) used for rinsing glassware, dilutions and preparation of solutions was obtained from a Milli-Q water purification system (Millipore, Germany).
Preparation of sorbent
Rice husk collected from a local rice mill was grounded and washed with ultrapure water several times to eliminate impurities and to obtain constant pH before dried in an oven at 105 ºC for 24 h. The dried rice husk was sieved to obtain particles size between 300 to 600 µm and then heated in a furnace at 800 ºC for two hours. The thermally treated rice husk was then put in a desiccator for cooling before kept in a container for the adsorption experiments.
Preparation of solutions
Various concentrations of Zn(II) working solutions (30-180 mg/L) used for sorption experiments were prepared by dilutions of 1000 mg/L of Zn(NO3)2 standard solution with the ultrapure water. In order to adjust the pH of Zn (II) working solutions, 0.1 M of HCl solution was prepared by diluting 1.0 M of HCl solution, whereas 1.0 M NaOH solution was prepared by dissolving an accurate amount of sodium hydroxide pellet with the ultrapure water.
Sorption study
One hundred mL of 30 mg/L of Zn (II) solution which was adjusted to pH 6 with 1.0 M NaOH and /or 0.1 M HCl solutions, was mixed with 2 g of 300-600 µm particle size of thermally treated rice husk. The mixture was agitated at 150 rpm for 180 minutes at 30oC. After the contact time, the mixture was filtered using a 0.45 µm cellulose nitrate membrane filter. The content of Zn (II) ions content in the filtrate was analyzed using an inductively coupled plasma optical emission spectrometry (ICP-OES)(Perkin Elmer, OPTIMA 5300 DV). The sorption experiments were conducted in duplicates and repeated using 60, 90, 120, 150 and 180 mg/L of Zn (II) working solutions. The sorption capacity, qt , was determined using equation (1):

where Coand Ce were initial and final concentrations (mg/L) respectively, m is the mass of sorbent (g) and V is the volume of solution (L).
Results and Discussion
Adsorption isotherm models are applied to determine the interaction between adsorbent and adsorbate especially Langmuir and Freundlich isotherm models13. Hence, in this study, the equilibrium data were evaluated with these isotherm models. The coefficient of determination (R2) of the models were used to determine the best fitted isotherm model.
Langmuir isotherm model
Langmuir adsorption isotherm is widely used to describe the adsorption of organic and inorganic pollutants, such as dyes and heavy metals from aqueous solutions. It suggests that the adsorption onto the adsorbent surface is homogeneous, where the adsorption of solute from aqueous solution onto the adsorbent surface occurs as monolayer adsorption on the homogeneous number of exchanging sites14. Langmuir adsorption isotherm is expressed as below:
where qeis the amount of metals adsorbed on the sorbent (mg/g) at equilibrium, Ce is the concentration of metal solution at equilibrium, qmaxis the maximum sorption capacity describing a complete monolayer sorption (mg/g) and b is sorption equilibrium constant (L/mg) that is related to the free energy of sorption. The values of qmax and b were determined from the linear plot of Ce/qe versus Ce. Figure 1 shows the Langmuir plot for sorption of Zn (II). The data obtained with the coefficients of determination (R2) was presented in Table 1. Equilibrium parameter, RLis a dimensionless constant separation factor to express the essential features and characteristics of Langmuir isotherm15,16. RL can be defined as:
where b is sorption equilibrium constant (L/mg) that is related to the free energy of sorption and Co is initial concentration of Zn (II) ions (mg/L). The value of RL shows the sorption either unfavourable if RL > 1, linear if RL = 1, favourable if 0 < RL < 1, or irreversible if RL = 0. The RL values for the sorption of Zn (II) ions onto thermal treated rice husk using the lowest initial concentration (30 mg/L) and the highest initial concentration (180 mg/L) were 0.037 and 0.0064, respectively. The results proved that the sorption of Zn (II) ions using thermally treated rice husk is favourable process.
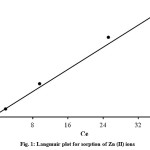 |
Figure 1: Langmuir plot for sorption of Zn (II) ions
|
Freundlich isotherm model
Freundlich isotherm model assumed that the adsorption process occurs from the interaction of the pollutant molecules on the heterogeneous surfaces. When the occupied binding sites increased, the energy of adsorption declined logarithmically14. Freundlich isotherm model used to evaluate the experimental was expressed by the following equation17.

where Kf is the Freundlich isotherm constant (mg/g) related to the bonding energy. Kf and n are constants and determined from the log qe versus log Ce plot. The constants Kf and n were calculated using Freundlich plot as shown in Figure 2. The values for Freundlich constants and coefficient of determination (R2) for the sorption process are presented in Table 1. The value of n between 1 and 10 (1/n less than 1) indicates favourable adsorption16. From Table 1, the value of n is 4.8497 indicating that the sorption of Zn (II) is favourable.
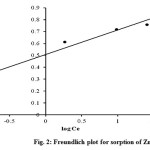 |
Figure 2: Freundlich plot for sorption of Zn (II)ions |
From Table 1, the results showed that the sorption of Zn (II) onto thermally treated rice husk was better fitted to Langmuir isotherm model (R2 = 0.9884) compared to Freundlich isotherm model (R2= 0.8496). These results proved that the sorption is monolayer with an equal activation energy and no interaction between sorbates on the adjacent active sites. Study by Elham et al.13 found that adsorption behaviour of Zn (II) onto rice husk was well described by Langmuir isotherm model, while sorption of Zn (II) by chemically prepared rice husk also follow Langmuir isotherm model10.
Table 1: Langmuir and Freundlich sorption isotherm constants for Zn (II) ions
| Langmuir constants | Freundlich constants | ||
| qmax (mg/g) | 6.5574 | Kf ((mg/g)/(mg/L)1/n) | 3.2181 |
| b (L/mg) | 0.8592 | n | 4.8497 |
| R2 | 0.9884 | R2 | 0.8496 |
Conclusion
In this work, mechanisms of Zn (II) ions sorption were proposed based on equilibrium data obtained. The sorption was better described by Langmuir isotherm compared to Freundlich isotherm which suggests that the sorption of Zn (II) ions onto the sorbent surface is on monolayer. The sorption process was favourable as indicated by the separation factor (RL) value. Thermally treated rice husk can be a candidate of cost effective sorbent to remove Zn (II) ions from wastewater. This approach also might help to solve the problem of agricultural waste management.
Acknowledgment
The authors are grateful to Universiti Pertahanan Nasional Malaysia for the support to conduct this research work.
References
- Bilal, M.; Shah, J. A.; Ashfaq, T.; Gardazi, S. M. H.; Tahir, A. A.; Pervez, A.; Haroon, H.; Mahmood, Q.; Journal of Hazardous Materials. 2013, 263, 322- 333.
CrossRef - Malamis, S.; Katsou, E.; Journal of Hazardous Materials. 2013, 252- 253, 428- 461.
- Mali, A.; Pandit V.; Majumder, D. R.; International Journal of Technical Research and Applications. 2014, 2, 42-46.
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh M.; Syouf, M. Q. A.; Journal of Environmental Chemical Engineering. 2015, 3, 775-784.
CrossRef - Regina, M. Y.; Saraswathy, S.; Kamal, B.; Karthik, V.; Muthukumaran, K.; Journal of Chemical and Pharmaceutical Sciences. 2015, 8, 1-6.
- Paduraru, C.; Tofan, L.; Teodosiu, C.; Bunia, I.; Tudorachi, N.; Toma, O.; Process Safety and Environmental Protection. 2015, 94, 18-28.
CrossRef - Oliveira, W. E.; Franca, A. S.; Oliveira, L. S.; Rocha, S. D.; Journal of Hazardous Materials. 2008, 152, 1073-1081.
CrossRef - Buasri, A.; Chaiyut, N.; Tapang, K.; Jaroensin, S.; Panphrom, S.; APCBEE Procedia. 2012, 3, 60-64.
CrossRef - Ong, K. K.; Md Nor, M. A; Mohamad, S; Ahmad Nasaruddin, N; Jamari, N. A.; Wan Yunus, W. M. Z.; AIP Conf. Proc. 2015, 1660, 070055.
CrossRef - El-Shafey, E. I.; Journal of Hazardous Materials. 2010, 175, 319-327.
CrossRef - Abdolali, A.; Ngo, H. H.; Guo, W.; Zhou, J. L.; Du, B.; Wei, Q.; Wang, X. C.; Nguyen, P. D.; Bioresource Technology. 2015, 193, 477-487.
CrossRef - Sadeek, S. A.; Negm, N. A.; Hefni, H. H. H.; Wahab, M. M. A.; International Journal of Biological Macromolecules. 2015, 81, 400-409.
CrossRef - Elham, A.; Hossein, T.; Mahnoosh, H.; J. Appl. Sci. Environ. Manage. 2010, 14, 159-162.
- Safa, Y.; Biosorption of Direct Dyes from Aqueous Solution Using Rice (oryza sativa) Husk, Vol. Doctor of Philosophy University of Agriculture, Faisalabad, 2010.
- Dada, A. O.; Ojediran, J. O.; Olalekan, A. P.; Advances in Physical Chemistry. 2013, 2013, 1-6.
CrossRef - Naiya, T. K.; Bhattacharya, A. K.; Mandal, S.; Das, S. K.; Journal of Hazardous Materials. 2009, 163, 1254-1264.
CrossRef - Freundlich, H.; Phys. Chem. Soc. 1906, 40, 1361–1368.

This work is licensed under a Creative Commons Attribution 4.0 International License.









