Physicochemical characteristic of BaTi0.95Zr0.05O3
Lamiae Mrharrab1*, Abdelhalim Elbasset2 and Salaheddine Sayouri1
1LPTA Department of Physics, University Sidi Mohamed Ben Abdellah, Faculty of SciencesD-M, B.P.1796, Fes-Atlas Morocco.
2LSSC Department of Electrical Engineering, Faculty of Science and Technology (FST), University Sidi Mohamed Ben Abdellah, Fes, Morocco.
Corresponding Author E-mail: mrharrab_lamiae@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/320509
Physical and chemicalproperties of BaTiO3 (BT) and BaTi0.95Zr0.05O3 (BZT0.05) ceramics have been studied. Thesepowders are prepared by the Sol-gel process and calcinedatdifferenttemperatures [500°C-1000°C]. X-Ray Diffraction (XRD) showed a crystallization of BT and BZT0.05 powders in the perovskite phase after a calcination temperatureat 600 °C, during 4 hours, but in the presence of some traces of zirconium oxide for the dopedpowder. Also, the effect of zirconium addition on dielectricproperties to BT isanalyzed. Indeed, dielectricmeasurementscarried out have shown the presence of a second anomaly for Zr dopedmaterialat 60 °C; an anomalythat has been attributed to structural transition.
KEYWORDS:Sol-gel Process; XRD; DielectricMeasurements; Anomalies
Download this article as:| Copy the following to cite this article: Mrharrab L, Elbasset A, Sayouri S. Physicochemical characteristic of BaTi0.95Zr0.05O3. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Mrharrab L, Elbasset A, Sayouri S. Physicochemical characteristic of BaTi0.95Zr0.05O3. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21919 |
Introduction
Barium titanium oxide, BaTiO3 (BT), was discovered for the first time during World War II in 19411. This compound which crystallizes in the perovskitestructure was widely used because it’senhanced dielectric permittivities2. BT presents a transition temperature of about 120 °C3, and for T Tc, it shows a tetragonal structure, with a ferroelectric behavior. It is also known that the dielectric properties are granulo-dependent4.
Substitution of another element in a pure matrix produces remarkable effects, either on structural characteristics such as crystalline phase, grain size and number of active phonon modes, or on physicochemical characteristics such as dielectric properties, dielectric constant, transition temperature and dielectric loss5-6-7-8.
There is various method of preparation of the ceramic or the powder fine perovskite9.We chose to work with the sol-gel method since it is known that this method produces powder with small grain sizes10.
In this work, we focus on the role of zirconium substituted in the BT material. Physical and chemical properties are studied. We worked on a percentage of 5% of Zr; BaTi0.95Zr0.05O3 (BZT0.05) material.
With the help of X-ray diffraction (XRD), we have studied the effect the Zr on the temperature crystallization and on the quality of the prepared powder.The dielectric measurements of sintered pellets are carried out between 30 to 200 °C, with the HP4284A impedance measuring bridge having a frequency range of 100 Hz to 2 MHz. The parallel faces of the samples are covered with electrodes conductive silver lacquer to form a parallel plate capacitor.The dielectric tests also showed the influence of Zr which is noted on several parameters that we will discuss later.
Procedure
The following precursors were used to prepare the BTZ0.05powders: barium acetate (Ba(CH3CO2)2; 3H2O, purity 99:9%, Johnson Matthey GmbH Alfa, Karlsrube, Germany), zirconium acetate (Zr(CH3CO2)4; xH2O) (purity 99.9%, x = 1.5, Johnson Matthey GmbH Alfa, Karlsrube, Germany), and titanium alcoxide (97 % (Assay) Johnson Matthey GmbH Alfa, Karlsrube, Germany). Distilled water was used as solvent. Preparation of the samples was needed two steps. The first step consisted of a preparation of a colloidal solution of TiO2. Titanium alcoxide was added to 0.5 M lactic acid aqueous solutions. A white precipitate was then obtained under stirring, at 60 °C, during 20 hours, which transforms into a clear solution. The latter was adjusted, by adding water, to 1M the solution. A rapid stirring was necessary to obtain a homogeneous solution (colloidal solution). In a second step were added to the latter colloidal solution, in stoichiometric amounts, barium and zirconium acetates as we have shown in other work11. Phase identification of the samples was performed using X-ray diffraction (Cu K ray, λ = 1.5418 Å). Dielectric measurements were carried out in the frequency range of 100 Hz to 2 MHz using an inductance capacitance resistance (LCR) bridge (HP, Model 4284 A).
Results and discussion
The first difficulty was to find the proper calcination temperature value for the complete crystallization, and without any secondary phase, for powder to prepare, which is verified by XRD analyzes.
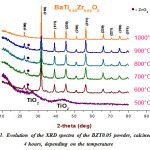 |
Figure 1: Evolution of the XRD spectra of the BZT0.05 powder, calcined for 4 hours, depending on the temperature |
Figure. 1 shows the XRD spectra of the sample BTZ0.05 calcined at different temperatures [500 °C to 1000 °C for 4 hours].These spectra clearly show the effect of increasing temperature on the quality of the prepared powder. In fact, the XRD pattern of the calcined powder at 500 °C is substantially amorphous, apart diffuse diffraction peaks and intense which was identified as peaks of reflection characteristics of the TiO2 precursor, no crystalline phase is apparent. However, at 600 °C the peak characteristic of the reflection phase TiO2 decreases almost completely and also registers the birth of the characteristic reflection peaks of BTZ0.05 phase. Toowe noted that the powder is completely crystallized into the perovskite phase, at 600 °C, with the presence of a secondary phase (ZrO2) at 2θ » 24°, this crystallization temperature is significantly lower than other processes like classical pathway and the solid coprecipitation12-13-14and also for sol-gel preparation method15.On the other hand, the intensity of the secondary phase decreases with increasing calcination temperature. Thereby, powder calcined at 1000 °C for 4 h, led to a solid solution BTZ0.05pure and without secondary phase.Also we observed, from Fig.2, the crystallization peak of the perovskite phase becoming narrower when the calcination temperature increases and the reflection peaks of ZrO2 phase disappears completely.This shows a better crystallization of the phase.
Even if,we see that the spectrum of the powder calcined at 1000 °C, for 4 h, is virtually a reproduction of the XRD spectrum corresponding to the calcined powder at 600 °C. However, from Fig.2, there is a displacement of the position of the peak (110) to the highest angles, reflecting the effect of the calcination temperature on the crystallization of the BTZ0.05 powder.
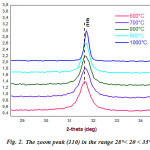 |
Figure 2: The zoom peak (110) in the range 28°< 2θ < 35° |
In order to make a comparison of the XRD spectra of the pure BT powder calcined at the same temperature values that the BTZ0.05 sample, we proceed to develop a pure BT powder (Fig.3).Fig. 3 shows that, BT spectra are isotype of that BTZ0.05.Indicating that pure perovskite phase, without the presence of secondary phase, is fully attained at the calcination temperature 600 °C.However,it may be noted two major differences between powders BT and BZT: First to 600°C, even 900°C,spectrum of BTZ0.05, also shows the presence of the ZrO2 phase while for BT the secondary phase (TiO2) has completely disappeared at 600 °C,indicating that the zirconium doping increases the calcining temperature of the pure perovskite phase.This is due to the atomic number of zirconium size which is larger or the nature of the bonds.These results are in good agreement with those found in the literature16-17. And second, With regard to the spectrum of the BT powder calcined at 600 °C, we see that the intensity of the reflection peaks, characteristics of pure perovskite phase,is smaller than that of BTZ0.05.This can be interpreted by the effect of atomic radius of Zr(RZr R Ti).
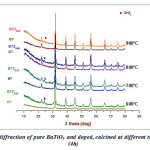 |
Figure 3: X-ray diffraction of pure BaTiO3 and doped, calcined at different temperature for (4h)Click here to View Figure |
In other words the substitution of Ti by Zr can increase the intensity peaks at certain crystallization temperatures.In the same way, to highlight the influence of zirconium on the structural state of BaTiO3 we calculated the lattice parameters a and c of BTZ0.05 by the use of CMPR program.The parameters a and c and their ratio c/a (quadracite) and their difference is illustrated in Table.1. Doping zirconium thus produced a reduction in quadracite and growthin volume. The Scherrer equation enabled us to determine the size of the powder grains PTZ0.05we found a value of 24.72 nm.
Table 1: Parameters of mesh and quadracite of BTZ0.05 (1000 °C (4h))
|
Rate Zr (%) |
Parameters a and c |
Quadracite (c/a) |
c-a (Å) |
Volume (Å3) |
|
|
x |
c (Å) |
a (Å) |
c/a |
||
|
0 |
4,0421 |
4,0014 |
1,0102 |
0,0407 |
64,7189 |
|
5 |
4,0423 |
4,0029 |
1,0098 |
0,0394 |
64,7718 |
The results of dielectric analyzes performed on pellets sintered at 1100 °C (8H) are shown schematically in Fig.4 .The shape of the dielectric constant as a function of the temperature of the pure sample (Fig.4.a) shows an increase in the dielectric constant values a function of the temperature to a maximum at a temperature of the order 150 °C with a maximum value of about 1900.This transition temperature is independent of the frequency;confirming thatit is the Curie ferroelectric-paraelectrictransition.The curves of Fig.4 .b and Fig.4.c of the dielectric permittivity which corresponds to the BZT0.05 show a similar trend to that observed in the case of BaTiO3 (pure sample), although the values of the dielectric permittivity measured at different frequencies are different. In addition, it is noted that the value of the transition temperature (Tc) decreases sharply. However, doping Zr produces a second anomaly at 60 °C (Fig.4 b). And the Fig.4.c shows the persistence of the second anomaly during cooling. Indeed, the work done by Baptisaet al.18we confirm that it is a structural transition and that such anomaliecan not be allocated to the vacant oxygen sites in the crystal structure. But recently Nanakornet al.14-15-19 have mounted that it is a orthorhombic-tetragonal structural transition (To-q).
 |
Figure 4: dielectric constant as a function of the temperature of the pure sample and of the BZT0.05 sample |
The choice of 1100 °C for 8 hours in the sintering step is not random, but after a study of function the sintering time. In fact, we prepared two samples one sintered at 1100 °C for 4 hours and the other at 1100 °C for 8 hours. The table.2shows the result of this study.It is thus observed that sintering at 1100 °C for 8 hours improves the dielectric constant obtained about 113% relative to the sintered for 4 h.
Table 2: Sintering time of effect on the dielectricproperties of BZT0.05
|
|
Sintering time (h) |
ε’max at Tc |
Tc |
|
BZT0.05 |
4 |
2976 |
130°C |
|
BZT0.05 (mounted) |
8 |
6740 |
120°C |
And on the other hand, to study the transport mechanism in BZT, we plotted the variation of the conductivity as a function of temperature under different frequencies.The conductivity was calculated from the measurements carried out, according to the relation below:
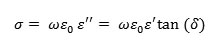
When the temperature increases, and below Tc, the conductivity follows the Arrhenius law:
![]()
With Ea the activation energy, T absolute temperature in Kelvin and KB is Boltzmann’s constant.The curves representing
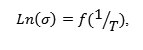
being straight (Fig.5) , the thermal activation energy can be easily calculated using different frequencies.The activation energy (Fig.6) shows an increase as a function of frequency in the frequency base region (f <100 kHz), followed by an almost linear increase in frequency above 100kHz. These values remain low confirming that the BTZ0.05 is a conventional ferroelectric material20.
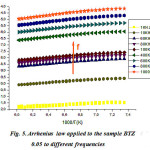 |
Figure 5: Arrhenius law applied to the sample BTZ 0.05 to different frequencies |
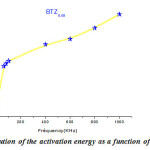 |
Figure 6: Evolution of the activation energy as a function of the frequency |
Conclusion
The addition of zirconiumproduced intense effectson properties of the pure powder BT, keeping the same phase as the latter,whatsoever on the value of proper calcination temperatureor on the quality and purpose of reflection peaks of the perovskite phase. More, there is an appearance of a second anomalie in the spectrum of the dielectric permittivity as a function of temperature for the sample doped with Zr,an anomalie attributed to the structural phase transition within the material under the effect of the temperature variation.This anomalie persists during the cooling step thus leaving the hypothesis prophesyit is not a question of the vacant oxygen sites. The measurements of the conductivity as a function of the temperature at different frequency values, confirms that pure BT and doped of Zr are conventional ferroelectrics materials.
Reference
- Randall, C.A.;Newnham,R.E. and Cross, L.E.“History of the First Ferroelectric Oxide, BaTiO3, Materials Research Institute”, The Pennsylvania State University, University Park, PA 16802 USA.
- Bertin,M.;Faroux,J.P. and Renault, J. Electromagnetisme 4, cours de physique, DunodEd.1994,.70.
- NENEZ, S.“Céramiquesdiélectriquescommandables pour applications micro-ondes: composites à base de titanate de baryum-Strontium et d’un oxyde non ferroélectrique”, Thèse de Doctorat, Université de Bourgogn2001.
- Bernaben, N.;leriche, A.;Thierry, B. and NiepceJ.C. Electroceramics1995,5, 203-210.
- Chi Man Lau, Xiao Wu Xu, K.W.KwokApplied Surface Science2015,336, 314-320.
- Li,W.L.;Cao,W.P.;Xu,D.;Wang,W.;Fei, W.D. Journal of Alloys and Compounds2014,613, 181-186.
- Yantao Liu, Wei Ren, Jinyan Zhao, Lingyan Wang, Peng Shi, Zuo-Guang YeCeramics International 2015, 41,( 1), 259-S264
- WanwilaiChaisan, RattikornYimnirun, SuponAnantaCeramics International 2009, 35(1), 121-124.
- Lamiae MRHARRAB, doctoral thesis,“Etude des propriétésphysico-chimiques de PbZrxTi1-xO3 (0 ≤ x ≤ 0,91) et de PbTiO3pur et dopé (V, Cu, Bi, Mn, Mg, La, Ca) élaborés par voiesolide”, University Sidi Mohammed Ben AbdellahMorocco2014.
- Abdelhalim ELBASSET,”SynthèseetCaractérisation de matériaux de Titanate de Baryumpurs et dopés au Strontium et au Zirconium”, Thèse de doctorat à la FST Maroc2014.
- Elbasset,A.;Lamcharfi, T.;Abdi, F.;Mrharrab, L. and Sayouri, S. Indian Journal of Science and Technology2015, 8(12), 56348
- Chen,H.;Yang, C.; Fu,C.;Shi,J.;Zhang, J.;Leng,W.J. Mater. Sci.2008, 19(4), 379-382.
- Kuang,S.J.;Tang,X.G.;Li,L.Y.;Jiang,Y.P.;Liu,Q.X. Scr. Mater.2009, 61, 68-71.
- Nanakorn,N.;Jalupoom,P.;Vaneesorn,N.;Thanaboonsombut,A. Ceram. Int.2008, 34, 779-782.
- Julphunthong,P.;Bongkarn,T. Current Applied Physics2011, 11(3), 60-65.
- B. Jaber, Thèse de l’université de Valencienneset du Hainaut-Cambresis1995.
- T. Lamcharfi, “Elaboration par voiehydrothermale et caractérisation des céramiquesferroélectriques de type pérovskite PZT dopé au lanthane Pb1-yLay(ZrxTi1-x)O3”,Doctorat national, UniversitéSidi Mohamed Ben AbdellahMaroc2005.
- Ang,C.;Yu,Z. and Cross,L.E. Phys. Rev. B2002, 62, 228-236.
- Kumar,M.;Garg,A.;Kumar,R. and Bhatnagar, M.C.Physica B: Condensed Matter2008, 403 (10), 1819-1823.
- Smolenskii,A.,”Ferroelectrics and Related Materials”,Gordon and Breach, New York1984, 589-606.

This work is licensed under a Creative Commons Attribution 4.0 International License.









