Mass Transfer Characteristics of Sodium alginate (SA) and Lignosulphonic acid (LS) Blends
S Giridhar Reddy and Akanksha Saxena Pandit
Chemistry Department, Amrita School of Engineering, Amrita Vishwa Vidyapeetham, Bengaluru Campus- 560035, Karnataka, India.
Corresponding Author E-mail: s_giri@blr.amrita.edu
DOI : http://dx.doi.org/10.13005/ojc/320553
Diffusion and sorption of Biodegradable blends of sodium alginate (SA) and lignosulphonic acid (LS) has been studied at room temperature by conventional weight gain experiments. The sorption studies of SA/LS blends are carried in pH medium of 7.4. The sorption data are used to evaluate the mass transfer coefficients such as intrinsic diffusion coefficient and permeation coefficient. It was observed that the blends follow Fickian mode of transport where polymer chain relaxation is more than diffusion of solvent.
KEYWORDS:Sodium alginate; Lignosulphonic acid; Sorption; Diffusion
Download this article as:| Copy the following to cite this article: Reddy S. G, Pandit A. S. Mass Transfer Characteristics of Sodium alginate (SA) and Lignosulphonic acid (LS) Blends. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Reddy S. G, Pandit A. S. Mass Transfer Characteristics of Sodium alginate (SA) and Lignosulphonic acid (LS) Blends. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=22648 |
Introduction
Diffusion of small molecules through the polymers has significant importance in different fields such as drug and pesticides delivery, packaging industries, membrane separation, extraction of solvents. Mass transfer with in a polymer depends on polymeric structure, type of crosslinking, penetrants, temperature and presence of fillers etc. Diffusion studies have been made on macromolecular system extensively1-3. Elastomers usually follow Ficks laws of diffusion while glassy polymers show no Fickian transport behavior.
Sodium alginate, a polysaccharide extracted from seaweeds, show excellent performance as a membrane material. It is a biodegradable, biocompatible, nontoxic, water soluble and gelling agent 4-10. Water solubility and poor mechanical weakness are the drawbacks of SA membranes for possible use in drug delivery applications. Biodegradable Polymer blends represents very important field in processing of new materials, which has better properties in comparison with the original polymers 11.
In our earlier studies, blends of SA and LS have been prepared and swelling properties of these blends have been studied for water uptake in a pH medium of 7.4 12-13. In the present work swelling properties of SA/LS blends with different compositions are studied. These blends are crosslinked using 2% CaCl2 solution for different time intervals. The water uptake in pH medium is recorded. Based on these swelling data diffusion and permeation parameters are evaluated for these blends.
Experimental
Chemicals
SA and LS obtained from Sigma-Aldrich, Bangalore, India Limited, and calcium chloride, buffer capsules of pH 7.4 are obtained from Nice Chemicals. Distilled water is used in all the experiments. These chemicals are used without any further purification.
Preparation of SA/LS Blends
The SA/LS films are formed by solvent casting method. The sample films obtained are dried at room temperature for 3 days; and to maintain constant weight they are further dried at 60 °C in vacuum oven. The films are cut in circular shape of 1.9±0.1cm 14.
Preparation of Ca2+ ions Crosslinked SA/LS Blends
Ca2+ ions crosslinked SA/LS blends are obtained by keeping the blends in 2% CaCl2 solution for different time intervals.
Characterization technique
The crosslinked blends are characterized for swelling behavior using weight gain method.
The Percentage of Swelling = (m2 – m1) / m1 × 100.
Where, m2 is final weight and m1 is initial weight.
Results and Discussions
Swelling behavior of SA/LS blends in pH 7.4 medium using 2% CaCl2 solution as crosslinking agent.
In order to investigate the effect of crosslinking agent on swelling properties of blends SA/LS blends of different compositions ( 100/0, 90/10, 80/20, 70/30 and 60/40 by weight) are crosslinked for 10, 20 and 30 min. These blends are then, investigated for their swelling behavior in buffer solution of pH 7.4 at temperature 27±0.5 oC. The sorption plots (molar percent versus square root of time in min) for all SA/LS blend composition are plotted in Fig.1 to Fig.5. All curves show initially almost a linear increase up to equilibrium swelling which shows the uptake of solvent by the blends increases with time. Later swelling does not happen and graph levels off. Experiments are continued for longer time to ensure the equilibrium swelling. Average of three trials is used for this study.
Swelling of Pure Sodium Alginate (100/0)
Pure SA crosslinked films are studied for swelling in a pH medium of 7.4. It is observed from Fig.1 that 10 and 20 min crosslinked films show very high water uptake (29±5%) and starts degrading / breaking after 30 and 40 min indicating poor crosslinking, whereas 30 min crosslinked film swells up to 28±3% in 30 min and continues to swell till it reaches reaches to equilibrium swelling in 1 h.
Swelling of SA/LS Blend (90/10)
The swelling behavior of SA/LS (90/10) blend films is shown in the Fig. 2. The blend crosslinked for 10 and 20 min swells 28±5%, and 21±3% in 1 h, whereas 30 min crosslinked blend swells up to 12±2%. It is observed from Fig. 2 that there is a reduction in swelling compared to pure SA films which indicates the improvement in physical strength after blending.
Swelling of SA/LS Blend (80/20)
The SA/LS (80/20) blends are prepared and their swelling behavior is shown in Fig. 3. It is observed that swellings are further controlled in this composition. The 10 min crosslinked blend swells about 25±4%, and swelling decreases drastically in 20 and 30 min crosslinking blends to 16.6±3% and 11.77±1% respectively in 1 h.
Swelling of SA/LS Blend (70/30)
On increasing LS content in the blends up to 30%, the blends were found to swell up to 19.25%, 15.66% and 11.27% in 1 h for 10, 20, and 30 min crosslinking respectively as shown in the Fig.4.
Swelling of SA/LS Blend (60/40)
For 40% LS content in the blend it was observed that these blends sometimes develop cracks and turn brittle and during swelling a fraction of LS dissolves into the aqueous solution. 10 min crosslinked blend swells up to 16.873%; whereas 20 and 30 min crosslinked blends swells 9.55% and 6.72% in 1 h (Fig.5).
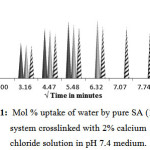 |
Figure 1: Mol % uptake of water by pure SA (100/0) system crosslinked with 2% calcium chloride solution in pH 7.4 medium.
|
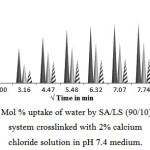 |
Figure2: Mol % uptake of water by SA/LS (90/10) blend system crosslinked with 2% calcium chloride solution in pH 7.4 medium. Click here to View Figure |
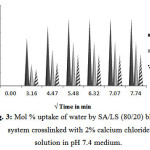 |
Figure 3: Mol % uptake of water by SA/LS (80/20) blends system crosslinked with 2% calcium chloride solution in pH 7.4 medium. Click here to View Figure |
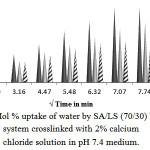 |
Figure 4: Mol % uptake of water by SA/LS (70/30) blends system crosslinked with 2% calcium chloride solution in pH 7.4 medium. Click here to View Figure |
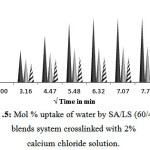 |
Figure 5: Mol % uptake of water by SA/LS (60/40) blends system crosslinked with 2% calcium chloride solution.
|
Mass Transfer Characteristics Of Sa/Ls Blends
The swelling behavior of various SA/LS blends are described in Fig.1 to Fig.5 which are quantitative in nature. But in order to understand mass transfer in SA/LS blends, sorption, diffusion and permeation are studied using the swelling results. The swelling of SA/LS blends of composition (100/0, 90/10, 80/20, 70/30 and 60/40) crosslinked for 10, 20 and 30 min using 2% CaCl2 solution in pH 7.4 medium are used for these studies.
Sorption Studies
To gain more insight in to mass transport phenomenon, the sorption results are fitted to heuristic expression [15-17].
log Qt/Q∞= log k+ n logt ——– (1)
where Qt mole % sorption at time t and Q∞ is sorption at equilibrium. The constant k depends on the polymer structure and polymer interaction with solvent and n denotes extent of mode of transport. The value of n = 0.5 for normal Fickian mode of transport where the rate of polymer chain relaxation is higher compared to the diffusion rate of the penetrant. When n =1, it is ‘relaxation controlled’ i.e. solvent diffusion is faster compared to chain relaxation. The mode of transport is said to be anomalous if the values of n is in between 0.5 and 1. The exponent n and k are calculated from slopes of sorption plots (Fig.1 to 5). The values of n and K for pure SA and SA/LS (90/10) blend are found to be less than 0.5. This could attribute to the fact that, the expression (1) is valid for only for short intervals of time and Qt/Q∞˂0.5 as given in references 15, 18. In our study the Qt/Q∞ > 0.5 where maximum swelling is occurring within 30 min. Hence we could not get satisfactory results from this equation. But the values are quite nearer to Fickian where the polymer chain relaxation is higher compared to diffusion rate of penetrant. However the values of n and k are increasing with higher composition of LS in SA/LS blends and they are found to be anomalous. The values of n and k are given in Table 1.
Diffusion and Permeation Studies
Based on sorption studies and since the sufficient reduction in swelling observed in SA/LS blends, the diffusion coefficient (D) of the polymer- solvent interaction has been calculated using Fickian model 19.
D= π (Xm/4 Q∞) 2 ———- (2)
where ‘X’ is the thickness of sample, ‘m’ is the slope in the sorption curve before attaining 50% of equilibrium and Q∞ is the mol % at equilibrium. The values D are furnished in Table 2. It has been observed that the D decreases with the SA/LS blend composition.
The permeation process through any matrix is a combination of diffusion and sorption and hence, it depends on these parameters. The calculation for P (permeation coefficient) for all the blends are done using the expression (3) 20.
P = DS ————– (3)
Sorption coefficient ‘S’ represents the ratio of the mass of the solvent molecule at equilibrium swelling to the mass of the polymer blend sample. The p values obtained are given in Table 2. It is observed that the penetration values of the blend–solvent systems follow the same trend as that of diffusion coefficient.
Table 1: The values of n and k for SA/LS blends of 100/0, 90/10, 80/20, 70/30 and 60/40 observed in pH 7.4 medium using 2% CaCl2 solution for different crosslinking timings.
|
Blend composition (SA/LS) |
Crosslinking timing (2% CaCl2) |
‘n’ |
‘k’ |
|
100/0 |
10 min |
0.38 |
0.39 |
|
20 min |
0.453 |
0.42 |
|
|
30 min |
0.46 |
0.64 |
|
|
90/10 |
10 min |
0.39 |
0.41 |
|
20 min |
0.46 |
0.48 |
|
|
30 min |
0.48 |
0.56 |
|
|
80/20 |
10 min |
0.42 |
0.35 |
|
20 min |
0.47 |
0.38 |
|
|
30 min |
0.49 |
0.47 |
|
|
70/30 |
10 min |
0.44 |
0.35 |
|
20 min |
0.48 |
0.36 |
|
|
30 min |
0.56 |
0.475 |
|
|
60/40 |
10 min |
0.51 |
0.62 |
|
20 min |
0.53 |
0.651 |
|
|
30 min |
0.58 |
0.75 |
Table 2: The values of Intrinsic Diffusion coefficient (D) and Permeation coefficient (P) at 27±0.5 oC. The values for 10 min crosslinking are not given because the sample starts degrading.
| Blend composition (SA/LS) | Crosslinking time (2% CaCl2) | D*x105 (cm2/s) | Px108 (cm2/s) |
| 100/0 |
10 min |
– |
– |
|
20 min |
8.75 |
28.1 |
|
|
30 min |
3.54 |
9.9 |
|
| 90/10 |
10 min |
– |
|
|
20 min |
2.22 |
4.7 |
|
|
30 min |
1.29 |
1.6 |
|
| 80/20 |
10 min |
– |
– |
|
20 min |
0.87 |
1.4 |
|
|
30 min |
0.42 |
0.49 |
|
| 70/30 |
10 min |
– |
– |
|
20 min |
0.42 |
0.78 |
|
|
30 min |
0.32 |
0.66 |
|
| 60/40 |
10 min |
– |
– |
|
20 min |
0.423 |
0.41 |
|
|
30 min |
0.153 |
0.12 |
Conclusion
Biodegradable polymer blends of SA and LS are prepared by solution casting method with various proportions of SA/LS (100/0, 90/10, 80/20, 70/30 and 60/40). The calcium ion crosslinked blends are subjected for swelling studies in pH medium of 7.4. It was observed, that the strength of blends in an aqueous medium is controlled by different crosslinking time. This attributes to the fact that controlling the strength of blends may influence the drug release. A comparative study revealed that SA/LS (80/20) blend films show moderate swellings and results obtained are quite consistent. The sorption studies of pure SA and SA/LS blends confirm that ‘n’ exponent increases on blending and its value is nearer to Fickian and tends towards anomalous behavior with increase of LS in SA/LS blends.
References
- Errede, L.A. Macromolecules. 1986, 654, 1525-1528.
- Chiou, J.S.; Paul, D.P. Polymer Engineering Science. 1986, 16, 1218-1227.
- Kraus, G.J, Journal of applied polymer science, 1963, 7, 861-871.
- Kim, S. W.; Bae Okano, T. Pharmaceutical Research. 1992, 9, 283-290.
- Gutowska, A.; Jeong Jasionowski, B. The Anatomical Record. 2001, 263, 342-349.
- Sakai,S.; Masuhara,H.; Yamada,Y.; Ono,T.; Ijima ,H.J. Bioscience and Bioengineering. 2005, 100, 127-129.
- Zimmermann, H.; Zimmermann, D.; Reuss, R. Journal of Materials Science: Materials in Medicine. 2005, 16, 491-501.
- Smidsrod ,O.; Skjak-Braek, G. Biotechnology. 1990, 8, 71-78.
- Jayachandran.; Venkatesan.; R, Rangasamy Jayakumar.; Sukumaran Anil.; P, Elna. Chalisserry.; Ramjee Pallela.; Se-Kwon Kim. Journal of Biomaterials and Tissue Engineering. 2015, 5, 458–464.
- Jayakumar, R.; Rajkumar, M.; Freitas, H.; Selvamurugan, N.; Nair, S,V ;Furuike, T.; Tamura, H. International Journal of Biological Macromolecules. 2009, 44, 107–111.
- Buthaina, A.; Ibrahim.; Karrer Kadum, M. Modern Applied Science. September 2010, 4, 9-11.
- Giridhar Reddy, S.; Akanksha Saxena Pandit. Revista Polimeros -Ciencia e Technologia. 2013, 23(1), 13-18.
- Giridhar Reddy, S.; Akanksha Saxena Pandit. International Journal of Polymeric Materials and Polymeric Biomaterials. 2013, 62(14), 743-748,.
- Travinskaya, T.V.; Savelyev, Y.V. European Polymer Journal. 2006, 42, 388-394.
- Shivaputrappa, B.; Harogoppad.; Tejraj Aminabhavi, M. Macromolecules. 1991, 24, 2598-2605.
- Sujith, A.; Radhakrishnan, C.K.; Unnikrishnan,G.; Sabu Thomas. Journal of Applied Polymer Science. 2003, 90, 2691-2702.
- Soney Jose.; Sabu Thomas. Progress in Polymer Science. 2001, 26, 985-1017.
- Erdener Karadag. Polymer-Plastics Technology and Engineering. 2006, 45, 729-734.
- Harogoppad, S.B.; Tejraj Aminabhavi, M. Journal of Applied Polymer Science. 1991, 42, 2329-2336.
- Cassidy,P.E.; Tejraj Aminabhavi ,M.; Thompson.C.M. Rubber Chemistry and Technology. 1983, 56, 594-618.

This work is licensed under a Creative Commons Attribution 4.0 International License.









