Highly Efficient Synthesis of New 2H-Chromene Dyes using Cu-SBA-15
Shirin Faraji Kamazani and Siavash Salek Soltani
School of Chemistry, University College of Science, University of Tehran, Tehran, Iran.
Corresponding Author E-mail: Shirinfaraji58@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320525
In this work, some new azotated 2H-chromene derivatives were successfully synthesized by use of mesoporous Cu-SBA-15 as nanocatalyst leading short reaction times and high yields. By this research, the scope of azo compounds was increased.
KEYWORDS:Azo dye; Chromene; Mesoporous; Catalyst; Cu-SBA-15
Download this article as:| Copy the following to cite this article: Kamazani S. F, Soltani S. S. Highly Efficient Synthesis of New 2H-Chromene Dyes using Cu-SBA-15. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Kamazani S. F, Soltani S. S. Highly Efficient Synthesis of New 2H-Chromene Dyes using Cu-SBA-15. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21255 |
Introduction
2H-chromenes are very important heterocyclic compounds which have shown biological activities1-4, some medical properties such as antihypertensive5 and cardio protectors as well as hypoglycemic agents6,7.Some of them are insecticide compounds8.In the recent years, they have been used in sensors for detection of metals and some compounds with high selectivity9,10.Until now, diverse methods have been published for the synthesis of 2H-benzopyran derivatives11-17.They are also good intermediates that have been applied in several organic synthesis18-20.Azo compounds are very considerable in dyes and pigments industry21.They are the main class of all synthetic dyes that are widely used in the world22.The importance of azo dyes returns to their significant physicochemical features such as stability23,optical properties24,25and their extensive applications in chemosensors26,27,liquid crystals28 and nanotubes29.Recently, an enhancing attention has been concentrated on the development of new synthetic paths in processes employing functionalized mesoporous catalysts30.
Mesoporous material has been grown in academia because of its extraordinary surface area, narrow size distribution of porosity, heat resistance and mechanical stability that make them outstanding catalysts31,32. In this work, we use them for the synthesis of new class of azo dyes in very good conditions.
Experimental
General information
In this research, 5-Phenylazo-salicylaldehyde 1 was synthesized similar to the reported methods33, 34, but optimized, and also the mesoporous Cu-SBA-15 catalyst was prepared by literature35.
All of the chemicals and solvents were purchased from Merck (Germany), Sigma-Aldrich (United States) and Fluka (Switzerland) corporations. They were used without anymore purification. TLC was performed on precoated plastic sheets (25DCUV-254). Melting points totally were weighed on an electrothermal Gallenkamp melting point apparatus and are not corrected. Elemental analysis for C, H and N were done using a Thermo Finnigan Flash EA1112 instrument. IR spectra were obtained on a Shimadzu FT-IR-4300 spectrophotometer as KBr discs. 1HNMR and 13CNMR spectra were analyzed on a Bruker 300 MHz spectrometer (but 2a, 2c and 2d compounds were analyzed on a Bruker 250 MHz spectrometer) in CDCl3, and DMSO- d6, solutions and chemical shifts were noted in δ units by using TMS, as standard.
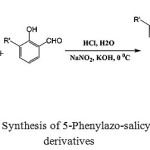 |
Scheme 1: Synthesis of 5-Phenylazo-salicylaldehyde derivatives |
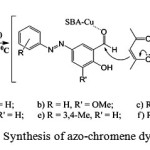 |
Scheme 2: Synthesis of azo-chromene dyes |
General procedure for the synthesis of 5-Phenylazo-salicylaldehyde (1)
To a solution of substituted aniline (0.05 mol) in a small portion of water was slowly added 7 ml of 37% aq HCl solution at 0-5 0C with rapid stirring. 15 ml of 5 M aq NaNO2 solution was added dropwise to afford yellowish diazonium salts. Then the solution of salicylaldehyde (3-methoxy salicylaldehyde, 0.05 mol) in 2% aq KOH solution (100 mL) was added very slowly in 1 hour. The mixture was stirred for 30 minutes. The brown solid was filtered and washed with sufficient water. Finally it was recrystallized from ethanol to get the pure product.
Synthesis of 1-(2-Hydroxy-2-methyl-6 (phenyldiazenyl)-2H-chromene-3-yl) ethanone (2a).
In the mixture of 5-phenylazo-salicylaldehide 1a (1.13 g, 5 mmol) and acetylacetone (0.52 g, 5 mmol) in 25 ml solvent (CH2Cl2/EtOH; 2:1), the catalyst Cu-SBA-15 (5 mol%) was added under rapid stirring in ice bath. The reaction mixture was stirred for 1 hour in 0 oC. The solution was remained in refrigerator for 12 hours. Then, the solvent was evaporated with vacuum rotary. Afterward, the precipitate was washed with sufficient n-hexane. In order to recovery of the catalyst, the product was dissolved in acetonitrile (10 ml) and shaken for 5 min. then the catalyst was filtered and washed with enough ethyl acetate and the filtrate was evaporated to gain crude product. It was recrystallized from ethanol to obtain orange pure crystals, mp, 134-136 oC. The similar procedure was used leading 2b-f.
Representative spectral data
1-(2-Hydroxy-2-methyl-6-(phenyldiazenyl)-2H-chromene-3-yl) ethanone (2a)
IR (KBr) (νmax, cm−1): 3473, 1653, 1431, 1163, 1083. 1HNMR (250 MHz, DMSO): δH 1.87 (3H, s, CH3), 2.47 (3H, s, OCH3), 4.4 (1H, brs, OH), 7.07 (1H, d, J = 8.75 Hz, H8), 7.46-7.87 (7H, Ar Protons), 7.97 (1H, dd, J = 8.5 Hz, J = 2.5 Hz, H7). 13CNMR (62 MHz, DMSO): δC 32.5 (CH3), 32.6 (CH3), 111.3, 122.5, 125.1, 127.6, 128.6, 131.9, 134.7, 136.3, 139.2, 140, 151.5, 157.2, 161.2 (Alkene and Aryl carbons), 201.6 (C=O).
1-(2-Hydroxy-8-methoxy-2-methyl-6-(phenyldiazenyl)-2H-chromene-3-yl) ethanone (2b)
IR (KBr) (νmax, cm−1): 3472, 1665, 1431, 1122, 1072. 1HNMR (300 MHz, DMSO): δH 1.89 (3H, s, CH3), 2.42 (3H, s, OCH3), 3.32 (1H, brs, OH), 3.89 (3H, s, OCH3), 7.72 (1H, s, H4), 7.52-7.89 (7H, Ar Protons). 13CNMR (75 MHz, DMSO): δC 33.1 (CH3), 32.9 (CH3), 59.7 (OCH3), 111.1, 113.7, 127.1, 127.9, 132.2, 133.8, 135.5, 139.6, 141.1, 149.8, 154.1, 155.2, 156.1 (Alkene and Aryl carbons), 200.3 (C=O).
1-(2-Hydroxy-2-methyl-6-(p-tolyldiazenyl)-2H-chromene-3-yl) ethanone (2c)
IR (KBr) (νmax, cm−1): 3383, 1664, 1422, 1142, 1077. 1HNMR (250 MHz, DMSO): δH 1.85 (3H, s, CH3), 2.42 (3H, s, CH3), 2.85 (3H, s, CH3), 3.1 (1H, brs, OH), 7.10 (1H, d, J = 9 Hz, H8), 7.36 (2H, d, J = 8 Hz, H10), 7.81 (2H, d, J = 7.75 Hz, H9), 7.91-7.93 (2H, m, H4,7), 8.03 (1H, d, J = 2.5 Hz, Hb). 13CNMR (62 MHz, DMSO): δC 25.7 (CH3), 32.9 (CH3), 33.2 (CH3), 110.8, 123.0, 125.1, 126.9, 128.2, 132.6, 134.0, 135.5, 139.8, 140.3, 149.7, 156.5, 160.7 (Alkene and Aryl carbons), 201.9 (C=O).
Table 1
| Cmpd |
Yields (%) |
mp (0C) |
Elemental Analysis (Found) |
Formula (M.W.) |
||
|
C H N |
||||||
|
2a |
88 |
134-136 |
70.12(70.11) |
5.23(5.19) |
9.09(9.02) |
C18H16N2O3(308.33) |
|
2b |
83 |
141-143 |
67.44(67.43) |
5.36(5.31) |
8.28(8.30) |
C19H18N2O4(338.36) |
|
2c |
85 |
128-130 |
70.79(70.77) |
5.63(5.55) |
8.69(8.65) |
C19H18N2O3(322.36) |
|
2d |
79 |
121-123 |
71.41(71.44) |
5.99(5.97) |
8.33(8.32) |
C20H20N2O3(336.38) |
|
2e |
81 |
145-147 |
71.41(71.40) |
5.99(5.95) |
8.33(8.30) |
C20H20N2O3(336.38) |
|
2f |
76 |
152-154 |
68.84(68.81) |
6.05(5.99) |
7.65(7.59) |
C21H22N2O4(366.41) |
Yields, mps and Elemental Analysis of 2a–f
1-(6-((4-ethylphenyl)diazenyl)-2-hydroxy-2-methyl-2H-chromene-3-yl) ethanone (2d)
IR (KBr) (νmax, cm−1): 3291, 1654, 1411, 1141, 1078. 1HNMR (250 MHz, DMSO): δH 1.23 (3H, t, J = 7.5 Hz, CH3), 1.87 (3H, s, CH3), 2.44 (3H, s, CH3), 2.70 (2H, q, J = 7.5 Hz, CH2), 3.1 (1H, brs, OH), 7.10 (1H, d, J = 8.75 Hz, H8), 7.42 (2H, d, J = 8.25 Hz, H10), 7.79 (2H, d, J = 8.25 Hz, H9), 7.90-7.94 (2H, m, H4,7), 8.03 (1H, d, J = 2.25 Hz, H5). 13CNMR (62 MHz, DMSO): δC 16.3 (CH2), 30.6 (CH3), 32.6 (CH3), 32.8 (CH3), 111.5, 122.2, 124.6, 126.8, 128.7, 132.0, 133.8, 135.5, 140, 140.5, 150.4, 156.1, 159.9 (Alkene and Aryl carbons), 201.7 (C=O).
1-(6-((3,4-dimethylphenyl)diazenyl)-2-hydroxy-2-methyl-2H-chromene-3-yl) ethanone (2e)
IR (KBr) (νmax, cm−1): 3444, 1659, 1440, 1165, 1079. 1HNMR (300 MHz, DMSO): δH 1.86 (3H, s, CH3), 2.28 (3H, s, CH3), 2.30 (3H, s, CH3), 2.42 (3H, s, CH3), 3.4 (1H, brs, OH), 7.08 (1H, d, J = 8.7 Hz, H8), 7.32 (1H, d, J = 8.1 Hz, H10), 7.58-7.90 (4H, m, Ar Protons), 7.99 (1H, d, J = 1.2 Hz, H5). 13CNMR (75 MHz, DMSO): δC 22.1 (CH3), 22.3 (CH3), 33.5 (CH3), 33.9 (CH3), 111, 119.5, 120.4, 122.5, 125.7, 127.3, 128.5, 132.6, 133.7, 135, 139.3, 139.7, 148.9, 156.1, 160.2 (Alkene and Aryl carbons), 201.1 (C=O).
1-(6-((4-ethylphenyl)diazenyl)-2-hydroxy-8-methoxy-2-methyl-2H-chromene-3-yl) ethanone (2f)
IR (KBr) (νmax, cm−1): 3456, 1662, 1401, 1127, 1076. 1HNMR (300 MHz, DMSO): δH 1.21 (3H, t, J = 7.5 Hz, CH3), 1.89 (3H, s, CH3), 2.42 (3H, s, CH3), 2.68 (2H, q, J = 7.8 Hz, CH2), 3.3 (1H, brs, OH), 3.88 (3H, s, OCH3), 7.40 (2H, d, J = 8.1 Hz, H10), 7.53 (1H, d, J = 1.8 Hz, H7), 7.69 (1H, d, J = 1.8 Hz, H5), 7.78 (2H, d, J = 8.1 Hz, H9), 7.87 (1H, s, H4).
Results and discussion
Diazotization of substituted aniline and a coupling reaction with 2-hydroxybenzaldehyde or its derivative (3-methoxysalicylaldehyde) yielded the corresponding 5-phenylazo-salicylaldehyde (Scheme 1).
Previously, an easy and rapid method for preparation of some 2H-chromene compounds was published36 and now we explain a simple and high efficiency pathway for the synthesis of azotated 2H-chromene (2), a novel class of dyes, using mesoporous Cu-SBA-15 as nanocatalyst (Scheme 2). Knoevenagel condensation of compound 1 with acetylacetone yielded compounds 2a–2f.
The structures of the new azo chromene dyes were deduced from their IR, 1HNMR, 13CNMR spectra, and also CHN analysis data. The 1H decoupled 13CNMR spectrum for each derivate is completely coincident with the product structure. Due to low solubility, we could not provide 13CNMR spectra for 2f compound.
Fig. 1 illustrates the number of carbon atoms for structure 2. It is helpful for 1HNMR interpretation in spectral data section.
The infrared spectra (IR) of these 2H-chromene dyes show a decreasing intense carbonyl bands due to conjugated carbonyl systems appearing at 1650–1670 cm−1. The broad bands in region of 3250–3480 cm−1 are referred to ν (O–H) vibrations. The peaks appearing at 1000–1200 cm−1 are attributed to ν (C–O) aromatic and aliphaticstretching vibration. The spectra also exhibit the existence of (–N=N–) group at 1400–1450 cm−1.
To explain the reaction mechanism, should focus on catalyst. The catalyst has lewis acid sites for polarizing carbonyl groups in both reagents, acetylacetone and 5-phenylazo-salicylaldehyde 1, via the nonbonding electrons on their oxygens. This coordination is more important for acetylacetone because it can lead the equilibrium to enol substance in a suitable solvent.
Since β–dicarbonyl compounds such as acetylacetone have keto-enol tautomerization, less polar solvents will move the equilibrium toward the enol form. In examine about effect of solvents, better yields were observed by using mixture of dichloromethane and low amount of a protic solvent. 2:1 volume ratio of dichloromethane and ethanol was most suitable according to the high yields (Table 1).
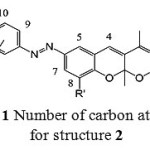 |
Figure 1: Number of carbon atoms for structure 2 |
The effect of temperature was also examined. When the reaction warm up to room temperature or higher, the efficiency will decrease. So using ice bath is necessary in whole of the reaction.
Conclusions
In summary, a new series of azo-chromene dyes 2a–f were synthesized by use of mesoporous nanocatalyst, Cu-SBA-15, leading short reaction times, very simple methodology, high efficiency, easy purification, minimal production of waste and performing reactions in the mild conditions. However, the diverse characteristics of these azo dye compounds need further investigations.
Acknowledgments
We gratefully acknowledge financial supports from the University of Tehran.
References
- Kang, Y.; Mei, Y.; Du, Y. G.; Jin, Z. Org. Lett. 2003, 5, 4481-4484.
CrossRef - Harel, D.; Schepmann, D.; Prinz, H.; Brun, R.; Schmidt, T. J.; Wünsch, B. J. Med. Chem. 2013, 56, 7442-7448.
CrossRef - Majumdar, N.; Paul, N. D.; Mandal, S.; Bruin, B.; Wulff, W. D. ACS Catal. 2015, 5, 2329-2366.
CrossRef - Li, B.; Pai, R.; Di, M.; Aiello, D.; Barnes, M. H.; Butler, M. M.; Tashjian, T. F.; Peet, N. P.; Bowlin, T. L.; Moir, D. T. J. Med. Chem. 2012, 55, 10896-10908.
CrossRef - Buckingham, R. E.; Clapham, J. C.; Hamilton, T. C.; Longman, S. D.; Norton, J.; Poyaer, R. H. J. Cardiovasc. Pharmacol. 1986, 8, 798-804.
- Lee, B. H.; Seo, H. W.; Yoo, S. E. Pharmacol. 2004, 70, 74-82.
CrossRef - Roberston, D. W.; Steinberg, M. I. J. Med. Chem. 1990, 33, 1529-1541.
CrossRef - US Patent, 4,716,238, Dec, 29 (1987), Chem. Abstr., 102 : 45779b (1985).
- Yue, Y.; Yin, C.; Huo, F.; Chao, J.; Zhang, Y. Sens. Actuator B-Chem. 2016, 223, 496-500.
CrossRef - Bekhradnia, A.; Domehri, E.; Khosravi, M. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 152, 18-22.
CrossRef - Dai, L.; Shi, Y.; Zhao, G.; Shi, M. Chem. Eur. J. 2007, 13, 3701-3706.
CrossRef - Yoshioka, E.; Kohtani, S.; Miyabe, H. Angew. Chem. Int. Ed. 2011, 50, 6638-6642.
CrossRef - Reddy, K. R.; Poornachandra, Y.; Dev, G. J.; Mallareddy, G.; Nanubolu, J. B.; Kumar, C. G.; Narsaiah, B. Bioorg. Med. Chem. Lett. 2015, 25, 2943-2947.
CrossRef - Xia, L.; Cai, H.; Lee, Y. R. Tetrahedron 2015, 71, 6894-6900.
CrossRef - Sairam, M.; Saidachary, G.; Raju, B. C. Tetrahedron Lett. 2015, 56, 1338-1343.
CrossRef - Kong, J.; Meng, T.; Su, J. Org. Process Res. Dev. 2015, 19, 681-683.
CrossRef - Paul, N. D.; Mandal, S.; Otte, M.; Cui, X.; Zhang, X. P.; Bruin, B. J. Am. Chem. Soc. 2014, 136, 1090-1096.
CrossRef - Zonouzi, A.; Izakian, Z.; Biniaz, M. Org. Prep. Proced. Int. 2009, 41, 543-548.
CrossRef - Costa, M.; Areias, F.; Abrunhosa, L.; Venancio, A.; Proenca, F. J. Org. Chem. 2008, 73, 1954-1962.
CrossRef - Roy, R.; Rakshit, S.; Bhowmik, T.; Khan, S.; Ghatak, A.; Bhar, S. J. Org. Chem. 2014, 79, 6603-6614.
CrossRef - Koukabi, N.; Otokesh, S.; Kolvari, E.; Amoozadeh, A. Dyes Pigm. 2016, 124, 12-17.
CrossRef - Nisal, A.; Trivedy, K.; Mohammad, H.; Panneri, S.; Gupta, S.; Lele, A.; Manchala, R.; Kumar, N. S.; Gadgil, M.; Khandelwal, H.; More, S.; Laxman, R. S. ACS Sustain. Chem. Eng. 2014, 2, 312-317.
CrossRef - Zhao, Y.; Ikeda, T. Smart light-responsive materials: azobenzene-containing polymers and liquid crystals. N.J: Wiley; 2009.
CrossRef - Shao, J. Dyes Pigm. 2010, 87, 272-276.
CrossRef - Blackburn, O. A.; Coe, B. J. Organometallics 2011, 30, 2212-2222.
CrossRef - Cheng, X.; Li, Q.; Li, C.; Qin, J.; Li, Z. Chem. Eur. J. 2011, 17, 7276-7281.
CrossRef - Li, T.; Yang, Z.; Li, Y.; Liu, Z.; Qi, G.; Wang, B. Dyes Pigm. 2011, 88, 103-108.
CrossRef - Bogdanov, A. V.; Vorobiev, A. K. J. Phys. Chem. B 2013, 117, 13936-13945.
CrossRef - Banerjee, I. A.; Yu, L.; Matsui, H. J. Am. Chem. Soc. 2003, 125, 9542-9543.
CrossRef - Kundu, S. K.; Mondal, J.; Bhaumik, A. Dalton Trans. 2013, 42, 10515-10524.
CrossRef - Dutta, A.; Mondal, J.; Patra, A. K.; Bhaumik, A. Chem. Eur. J. 2012, 18, 13372-13378.
CrossRef - Mondal, J.; Modak, A.; Dutta, A.; Bhaumik, A. Dalton Trans. 2011, 40, 5228-5235.
CrossRef - Li, J.; Li, X.; Wang, S. J. Mol. Struct. 2012, 1011, 19-24.
CrossRef - Manimekalai, A.; Balachander, R. J. Mol. Struct. 2012, 1027, 175-185.
CrossRef - Chen, L.; Guo, P.; Zhu, L.; Qiao, M.; Shen, W.; Xu, H.; Fan, K. Appl. Catal. A. Gen. 2009, 356, 129-136.
CrossRef - Zonouzi, A.; Googheri, M. S.; Miralinaghi, P. S. Org. Prep. Proced. Int. 2008, 40, 471-477.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









