Synthesis and Photophysical Properties of Rhenium(I)-Alkynyl Molecular Rectangles
Arumugam Ramdass,1 Veerasamy Sathish,2 Bala Manimaran3, Pounraj Thanasekaran,4 and Seenivasan Rajagopal*5,6
1Department of Chemistry, Aditanar College of Arts and Science, Tiruchendur – 628 216, India.
2Department of Chemistry, Bannari Amman Institute of Technology, Sathyamangalam, India.
3Department of Chemistry, Pondicherry University, Puducherry – 605 014, India .
4Institute of Chemistry, Academia Sinica, Taipei – 115, Taiwan.
5School of Chemistry, Madurai Kamaraj University, Madurai – 625 021, India.
6Department of Chemistry, Vivekananda College, Tiruvedagam West – 625 234, Madurai, India.
Corresponding Author E-mail: manimaran.che@pondiuni.edu.in
DOI : http://dx.doi.org/10.13005/ojc/320413
Article Received on :
Article Accepted on :
Article Published : 08 Aug 2016
Rectangles; alkynyl ligands; luminescence; quenching; rhenium
Download this article as:| Copy the following to cite this article: Ramdass A, Sathish V, Manimaran B, Thanasekaran P, Rajagopal S. Synthesis and Photophysical Properties of Rhenium(I)-Alkynyl Molecular Rectangles. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Ramdass A, Sathish V, Manimaran B, Thanasekaran P, Rajagopal S. Synthesis and Photophysical Properties of Rhenium(I)-Alkynyl Molecular Rectangles. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=19035 |
Introduction
In the natural photosynthetic apparatus, energy is harvested by antenna pigments and transferred into a reaction center where charge separation takes place1,2. Since the natural light-harvesting system contains a larger number of different chromophores, the artificial antennae require an ingenious molecular design that allows the incorporation of a number of different chromophore units and their efficient cooperation with one another, as well as acquiring light energy over a long distance to a designated unit. To mimic the antenna function, chemists have attempted to prepare different types of artificial systems3–10. Metallocyclophanes are of special interest since multiple chromophoric ligands can be incorporated into this system as found in photosynthetic reaction centers 8–10. As for an elegant example, Wurthner and co-workers10 demonstrated that a Pt(II)-based molecular square containing sixteen pyrene chromophores constitutes a light-harvesting system that undergoes the combination of rapid energy and efficient electron transfer processes.
Different from bimolecular complexes3–7 and molecular squares,8–10 rectangles contain two types of different chromophore units in the peripheral environments. In addition, transition metal-alkynyl compounds with a conjugated p-system have been a topic of rapidly growing interest as a result of their unique photo physical properties11–14. Yam and coworkers15 reported that the luminescence behavior of a rhenium(I) chromophore is perturbed by -C≡C- units. In addition, the alkynyl ligand exhibits an excellent conduit for electron transport, thereby facilitating fast intramolecular electron and energy exchange between the various subunits. With the objective of achieving desirable artificial antenna, we herein designed and synthesized a series of highly conjugated and rigid Re(I)-based molecular rectangles 1-3 containing two different types of bidentate pyridyl ligands. As shown in Chart 1, one of the ligand units is comprised of phenylene, naphthalene and anthracene, which is then connected by pyridyl ligands via an ethynyl spacer, forming the composite complexes 1, 2 and 3, respectively. Details are elaborated in the following section.
Materials and Methods
Materials
Reagents were used as received. All manipulations were performed under a nitrogen atmosphere using standard Schlenk techniques. Chromatographic separation could be done in air. Tetrahydrofuran was dried over CaH2 and was freshly distilled prior to use. Compounds 1,4-bis(4’-pyridylethynyl)benzene (bpeb), 1,4-bis(4’-pyridylethynyl)naphthalene (bpen) and 1,4-bis(4’-pyridylethynyl)anthracene (bpea) were synthesized by published methods16–18. The details of the synthesis of 1 have already been published19,20.
Instrumentation
IR spectra were recorded on a Perkin Elmer 882 FT-IR spectrophotometer and NMR spectra on a AMX-400 FT-NMR spectrometers. Elemental analyses were performed using a Perkin-Elmer 2400 CHN elemental analyzer. UV-visible spectra were obtained on a Hewlett Packard-8453 spectrophotometer at room temperature in 1-cm quartz cell. Fluorescence spectra were collected by means of a Hitachi F-4500 Fluorescence spectrophotometer. Luminescence decay measurements were performed with an Edinburgh single-photon counting instrument. The flash photolysis apparatus for the measurement of transient absorption spectra in the nanosecond time domain has been described elsewhere21. The excitation wavelength of 355 nm from a Nd:YAG laser (Continuum Surlite II, third harmonic) was used as the excitation source, coupled with a fast response photomultiplier (Hamamatsu model R5509-72) operated at -80 °C in nanosecond flash photolysis experiments (pulse width ca. 8 ns and energy 50 mJ per pulse). Transient spectra were obtained by a point-to-point technique, monitoring the absorbance changes (∆A) after flash at intervals of 10 nm over the spectral range 300-700 nm, averaging at least 30 decays for each wavelength. The values (the time at which the initial signal is t1/2 halved) are reported for transients showing second-order kinetics. Transient absorption signals were recorded using a laser flash photolysis system (Edinburgh LP920), in which an Nd:YAG laser (355 nm) pumped optical parametric oscillator and a white-light square pulse were used as the pump and probe beams, respectively. The temporal resolution was limited by an excitation pulse duration of ~8 ns. The low temperature emission spectra were taken using an Aminco Bowman series 2 luminescence spectrometer.
Syntheses of Rhenium(I)-based Molecular Rectangles
Synthesis of [{Re(CO)3(μ-bpy)Br}{Re(CO)3(μ-bpen)Br}]2 (2)
To a solution of {ReBr(CO)4}2(μ-bpy)22 (229 mg, 0.25 mmol) in CH2Cl2 (200 mL) and CH3CN (1 mL) at 5 °C was added a solution of Me3NO (38 mg, 0.51 mmol) in CH2Cl2 (30 mL). The reaction mixture was filtered through celite and the solvent was removed under vacuum and the residue was dissolved in CH2Cl2 (200 mL). To a solution of {ReBr(CO)3(NCMe)}2(μ-bpy) in CH2Cl2 a solution of 1,4-bis(4’-pyridylethynyl)naphthalene (bpen) (91 mg, 0.30 mmol) in CH2Cl2 (250 mL) was added slowly at 5 ºC. When the completion of the reaction was shown by IR spectroscopy, the solvent was evaporated and the product was subjected to chromatographic separation on a silica gel column by use of a mixture of acetone and CH2Cl2 (1:99) as eluent to afford compound 2 (121 mg, 0.051 mmol, 41%) as a greenish yellow solid. IR (CH2Cl2): nCO 2028 (vs), 1929 (s), 1896 (s) cm-1; 1H NMR (400 MHz, acetone-d6): d = 9.07 (d, 3J = 6.8 Hz, H3, 8 H, bpy), 8.91 (d, 3J = 6.6 Hz, H3, 8 H, bpen), 8.48 (dd, 3J = 6.4 Hz, 4J = 3.3 Hz, H5, 4 H, naphthyl, bpen), 8.05 (d, 3J = 6.8 Hz, H2, 8 H, bpen) 7.93 (s, H2, 4 H, naphthyl, bpen), 7.79 (d, 3J = 6.7 Hz, H2, 8 H, bpy), 7.73 (dd, 3J = 6.4 Hz, 4J = 3.1 Hz, H6, 4 H, naphthyl, bpen); 13C NMR (acetone-d6): d = 192.4, 196.7 (s, 2:1, CO), 156.3 (C3, bpy), 155.4 (C3, bpen), 147.0 (C1, bpy), 134.3 (C1, bpen), 133.6 (C4a, naphthyl, bpen), 132.0 (C2, naphthyl, bpen), 129.4 (C5, naphthyl, bpen), 128.6 (C2, bpen), 127.2 (C6, naphthyl, bpen), 124.8 (C2, bpy), 122.0 (C1, naphthyl, bpen), 95.5 (C2, ethynyl, bpen), 93.5 (C1, ethynyl, bpen); Anal. Calcd for C80H44N8O12Br4Re4•2.5(CH3COCH3): C, 42.05; H, 2.22; N, 4.41. Found: C, 41.95; H, 1.99; N, 4.20.
Synthesis of [{Re(CO)3(μ-bpy)Br}{Re(CO)3(μ-bpea)Br}]2 (3)
To a solution of {ReBr(CO)4}2(μ-bpy) (229 mg, 0.25 mmol) in CH2Cl2 (200 mL) and CH3CN (1 mL) at 5 ºC was added a solution of Me3NO (38 mg, 0.51 mmol) in CH2Cl2 (30 mL). The reaction mixture was filtered through celite and the solvent was removed under vacuum and the residue was dissolved in CH2Cl2 (200 mL). To a solution of {ReBr(CO)3(NCMe)}2(μ-bpy) in CH2Cl2 a solution of 1,4-bis(4’-pyridylethynyl)anthracene (bpea) (105 mg, 0.275 mmol) in CH2Cl2 (250 mL) was added slowly at 5 ºC. When the completion of the reaction was shown by IR spectroscopy, the solvent was evaporated and the product was subjected to chromatographic separation on a silica gel column by use of a mixture of acetone and CH2Cl2 (1:99) as eluent to afford compound 3 (118.7 mg, 0.048 mmol, 38%) as a bright red solid. IR (CH2Cl2): nCO 2028 (vs), 1929 (s), 1894 (s) cm-1; 1H NMR (400 MHz, acetone-d6): d = 9.13 (d, 3J = 6.7 Hz, H3, 8 H, bpy), 8.96 (d, 3J = 6.6 Hz, H3, 8 H, bpea), 8.73 (dd, 3J = 6.7 Hz, 4J = 3.2 Hz, H1, 8 H, anthracenyl, bpea), 8.11 (d, 3J = 5.6 Hz, H2, 8 H, bpea) 7.98 (d, 3J = 5.2 Hz, H2, 8 H, bpy), 7.74 (dd, 3J = 6.7 Hz, 4J = 3.2 Hz, H2, 8 H, anthracenyl, bpea); 13C NMR (acetone-d6): d = 196.4, 192.5 (s, 2:1, CO), 156.4 (C3, bpy), 155.5 (C3, bpea), 146.8 (C1, bpy), 134.0 (C1, bpea), 133.1 (C9a, anthracenyl, bpea), 128.8 (C2, bpea), 128.1 (C1, anthracenyl, bpea), 127.5 (C2, anthracenyl, bpea), 124.2 (C2, bpy), 118.8 (C9, anthracenyl, bpea), 100.0 (C2, ethynyl, bpea), 94.2 (C1, ethynyl, bpea); Anal. Calcd for C88H48N8O12Br4Re4•(CH3COCH3)9: C, 46.09; H, 3.43; N, 3.74. Found: C, 46.42; H, 2.99; N, 3.62.
Result and Discussion
The new molecular rectangles [{Re(CO)3(μ-bpy)Br} {Re(CO)3(μ-L)Br}]2 (2, L = 1,4-bis(4′-pyridylethynyl) naphthalene, bpen; 3, L = 1,4-bis(4′-pyridylethynyl)anthracene, bpea) were synthesized by following the synthetic procedure similar to the 1 used for the synthesis of compound [{Re(CO)3(μ-bpy)Br}{Re(CO)3(μ-L)Br}]2 (1, L = 1,4-bis(4′-pyridylethynyl)benzene, bpeb)19,20. Characterization of the complexes 1-3 was achieved by a combination of elemental analyses, IR, 1H and 13C NMR spectroscopies. The 1H NMR spectra for 2 and 3 are shown in Figs. S1 and S2, respectively.
 |
Figure S1: 1H NMR (400 MHz) spectrum of compound 2 in acetone-d6. Inset shows the expansion of aromatic region of 2. |
 |
Figure S2: 1H NMR (400 MHz) spectrum of compound 3 in acetone-d6. Inset shows the expansion of aromatic region of 3. Click here to View figure |
Absorption Spectra
The UV-vis absorption spectra of 1-3 were recorded in THF solution (Table 1). The intense high-energy absorption bands (230–310 nm) are ascribed to an admixture of ligand centered (LC) π → π* transitions of the bpy and alkynyl ligands as free ligands sit under these regions. Compared to the absorption bands of free ligands, the absorption bands in 1-3 are substantially red-shifted that could partially arise from the delocalization of both ligand-centered molecular orbitals due to the interaction with the Re(I) dπ orbital. Based on our previous reports by considering the redox potentials of free bpy and alkynyl ligands19, we tentatively assigned the peak at 323 nm to Re(I) → bpy and 348 nm to Re(I) → alkynyl. With reference to spectroscopic studies on related rhenium(I) alkynyl complexes15,19,23–25 and free pyridyl and alkynyl ligands, the molar extinction coefficients in 1-3 at their lowest absorption band are found to be higher (in the order of 104-105 dm3 mol–1 cm–1) than that of typical [dp(Re) → p*(L)] MLCT transition (usually in the order of 103 dm3 mol–1 cm–1). Hence, the absorption bands at 348, 385, and 465 nm for 1-3, respectively, are assigned to the [dπ(Re) → π*(alkynyl)] metal-to-ligand charge transfer (MLCT) transition, mixed with [π(alkynyl) → π*(bpy)] ligand-to-ligand charge transfer (LLCT) and intraligand [π → π*(alkynyl)] (IL) transitions (Fig. 1). Several rhenium(I)-bpy complexes with oligoether-appended coumarin (CM) ligands26 and pyridine containing macrocyclic phenylacetylene ligands27 have been shown to exhibit two MLCT bands, which are attributed to dπ(Re) → π*(bpy-CM)/dπ(Re) → π*(alkynyl) and dπ(Re) → π*(bpy)/dπ(Re) → π*(alkynyl) MLCT bands, respectively.
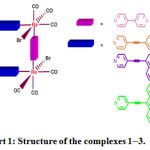 |
Figure : Structure of the complexes 1–3. |
Luminescence Spectra, Quantum Yields, and Lifetimes
All three complexes show luminescence upon excitation of their lowest absorption band. Emission color change from orange to blue and green in 1-3 mediated by the aryl substituents in the alkynylene, is observed for the first time. The blue shift in the emission maximum upon extending the p-conjugated system in these complexes represents an unusual phenomenon contradictory to the common concept of tuning the emission maximum to the red shift via extended conjugation28,29. This is tentatively attributed to either different perturbations of the singlet and triplet MLCT states by the aryl substituents or the excited state structure is probably more polar than ground states30,31. The broad and structureless emission band of 1 at 619 nm features a combination of both [dπ(Re) → π*(bpy)] and [dπ(Re) → π*(alkynyl)] 3MLCT characteristics, as Re(CO)3(bpy)2Cl (bpy = 4,4’-bipyridine) in CH2Cl2 featured an emission band at 585 nm that originated from [dπ(Re) → π*(bpy)] transition19, with an additional luminescence in shorter wavelength at 431 nm (Fig. 1). For the measurement of quantum yield calculation, emission band at 619 nm was employed. In contrast, complexes 2 and 3 display IL luminescence at 445 and 489(sh), 521 nm, respectively (Fig. 1, Table 1). i.e., the triplet CT Re-based excited states [dπ(Re) → π*(bpy)] and/or [dπ(Re) → π*(alkynyl)] in 2 and 3 undergo a quenching process, which can be attributed by triplet acceptors such as the naphthalene and anthracene moieties.
 |
Figure 1: Absorption and emission spectra of 1–3 (6 ´ 10-5 M) in THF. For each compound, the excitation wavelength is picked at the maximum of the lowest lying absorption band. Click here to View figure |
Table 1: Spectroscopic and photophysical data[a]
| Compounds | λabs , nm (emax, dm3 mol-1cm-1) | λemmax, nm | tem, ns | Fem (´ 10–3)[b] | kr × 103 s–1 | knr ´ 106 s–1 | λemmax, nm(77K) |
| bpeb | 229, 318, 377(sh) | 346, 361(sh) | 7.0 | —- | —- | —- | 367 |
| bpen | 237, 245, 278, 362, 380(sh) | 391, 412(sh) | 4.8 | —- | —- | —- | 415 |
| bpea | 230, 273, 309, 438, 465 | 474, 506 | 4.9 | —- | —- | —- | 509 |
| 1 | 233 (67 350), 254 (81 870), 348(125 670) | 431, 619 | 251 | 1.84 | 7.33 | 3.97 | 542, 574, 615(sh) |
| 2 | 234 (184 400), 256 (156 030), 274(sh), 385 (132 800) | 445 | 12.2, 13.0[c] | 0.54 | 44.3 | 82.0 | 417, 468 |
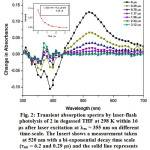 |
Figure 2: Transient absorption spectra by laser-flash photolysis of 2 in degassed THF at 298 K within 16 μs after laser excitation at lex = 355 nm on different time-scale. The Insert shows a measurement taken at 520 nm with a bi-exponential decay time scale (τem = 6.2 and 0.29 μs) and the solid line represents the exponential fitting of the data. Click here to View figure |
For excitation at any wavelength in the CT absorption region of 3, the structured emission maxima in the region 360-520 nm with a tail up to 700 nm can be observed at room temperature in steady-state emission spectra (Fig. S3). In consideration of the position, and the structured shape, the origin of these emission bands should be the luminescence of the LLCT and/or ILCT states in 3. The luminescence lifetimes for 2 and 3 are biexponential in the nanosecond time scale at room temperature, indicating the presence of two different excited states (Table 1). Hence, the observation of a small Stokes shift with short τem values in 2 and 3 indicates that emission is predominantly of 1π−π* character. In general, the emission maximum of 1-3 is sensitive to the nature of the alkynylene ligand. Low-temperature emission spectra for alkynylene ligands and 1-3 were recorded in a THF rigid matrix at 77 K (Figs. S4-S6), and the data are shown in Table 1. The excitation spectra of the emission in 2 and 3 were dependent of the emission wavelength selected and closely resembled the absorption spectra over the entire spectral range (Fig. S7).
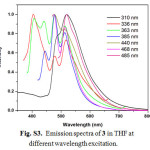 |
Figure S3: Emission spectra of 3 in THF at different wavelength excitation. Click here to View figure |
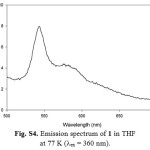 |
Figure S4: Emission spectrum of 1 in THF at 77 K (lex = 360 nm). Click here to View figure |
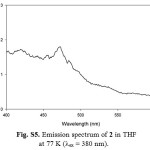 |
Figure S5: Emission spectrum of 2 in THF at 77 K (lex = 380 nm). Click here to View figure |
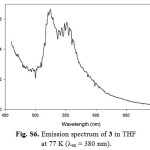 |
Figure S6: Emission spectrum of 3 in THF at 77 K (lex = 380 nm). Click here to View figure |
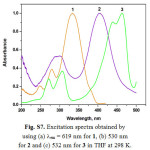 |
Figure S7: Excitation spectra obtained by using (a) lem = 619 nm for 1, (b) 530 nm for 2 and (c) 532 nm for 3 in THF at 298 K. Click here to View figure |
[a]Data in deaerated THF solution at room temperature; [b]Standard: Ru(bpy)32+ in CH3CN, Fem = 0.062 (J.M. Calvert, J.V. Casper, R.A. Binstead, T.D. Westmoreland, T.J. Meyer, J. Am. Chem. Soc. 104 (1982) 6620). [c]λemmax = 530 nm. [d]λemmax = 532 nm.
The photoluminescence lifetimes (τem) and quantum yields (Fem) for 1-3 in THF solution are listed in Table 1. The τem and Fem values were also used to compute the radiative and nonradiative decay rate constants (kr and knr respectively) and these parameters are compiled in Table 1. Inspection of the data for 1-3 collected in Table 1 reveal that there are no clear trends in either the τem or Fem values. The lack of a systematic trend in photophysical parameters for this series points out the possibility that more than one excited state contributes to the photophysics of these complexes. The most obvious among this series is the phenyl substituted complex 1, which features a knr that is nearly 20 times smaller than that of 2 and 10 times smaller than that of 3. The difference in non-radiative decay rates can be explained by the fact that the LLCT and/or ILCT states are close in energy to the MLCT states in 2 and 3. The excited state lifetime of 2 and 3 is increased slightly, compared to the free ligands bpen and bpea, respectively. This study also supports that part of the energy from the triplet donors, i.e., [dπ(Re) → π*(bpy)] and/or [dπ(Re) → π*(alkynyl)] chromophores, is transferred to triplet acceptors such as the naphthalene and anthracene moieties. The rate constant for energy transfer to the napthyl and anthryl units of 2 and 3, respectively can be obtained using eq 1.
![]()
where τem and τem(o) are the emission lifetime of 2 and 3 and model compound 1, respectively. Based on our observations, the energy transfer rate constants are 7.8 ´ 107 s–1 for 2 and 3.3 ´ 107 s–1 for 3. Additional support for the sensitization of the 3LLCT and/or 3ILCT states of 2 and 3 comes from transient absorption measurements.
Time-Resolved Absorption Spectra
By using nanosecond time-resolved spectroscopic techniques, insight can be gained into the excited state properties responsible for the photoinduced behavior of rectangles 1-3. The transient absorption (TA) spectrum of 1 exhibits a bleaching of the two MLCT absorptions in the region from 330 to 380 nm. In addition, this complex shows a broad and much stronger positive absorption at 420-700 nm (Fig. S8). The decay of the red absorption region is mono-exponential with a lifetime about 0. 320 ms, and the agreement with the emission lifetime of the low energy band in Fig. 1 suggests that they originate from the [dπ(Re) → π*(bpy)] and/or [dπ(Re) → π*(alkynyl)] 3MLCT excited states. Laser flash excitation of 2 at 355 nm leads to a structured bleaching of the MLCT absorption in the region from 330 to 430 nm with a maximum at 380 nm during triplet population32,33. In addition, this complex showed a broad and much stronger positive absorption at 450-700 nm (Fig. 2). The decay of the TA region is bi-exponential with a lifetime of t1 = 0.62 ms and t2 = 0.29 ms for 2. Since the free bpen alkynyl ligand also showed a stronger positive absorption at 480 nm with a decay of 0.85 ms (Fig. S9), we believe that the excited state decay of 2 originates from the different states, i.e., 3LLCT/3ILCT excited states. The transient absorption (TA) spectrum of 3 exhibits a bleaching of the MLCT absorption in the region from 420 to 480 nm with a maximum at 460 nm during triplet population32,33. In addition, this complex shows a broad and much stronger positive absorption at 500-700 nm (Fig. S10). The decay of absorbing triplet excited state is also bi-exponential with a lifetime of 2.44 ms and t2 = 0.011 ms for 3, which is not in agreement with the emission lifetime of the low energy band in Fig. 1 suggests that they originate from different excited states. Compared with the steady-state emission spectra of 2 and 3, the longer wavelength positive absorption band at 450-700 nm provides direct evidence for the existence of another excited state. The much longer lifetime for this band (t1 = 0.62 ms and t2 = 0.29 ms for 2 and 2.44 ms and t2 = 0.011 ms for 3) excludes the possibility of 1LLCT and 1ILCT excited states. The appropriate assignment for this triplet excited-state decays of 2 and 3 should be the 3LLCT and 3IL excited states.
 |
Figure S8: Transient absorption spectra by laser-flash photolysis of 1 in degassed THF at 298 K within 4 ms after laser excitation at lex = 355 nm in different time-scale. The bleaching at 350 nm is due to scattering of the laser light. Insert shows a measurement taken at 540 nm with a decay time scale (t = 0.32 ms) and the solid line represents the exponential fitting of the data. Click here to View figure |
 |
Figure S9: Transient absorption spectra by laser-flash photolysis of bpen in degassed THF at 298 K at 8 ms after laser excitation at lex = 355 nm on different time-scale. The ground-state absorption was ~0.7 at the excitation wavelength. Insert shows a measurement taken at 480 nm with a decay time scale (t = 0.85 ms) and the solid line represents the exponential fitting of the data. Click here to View figure |
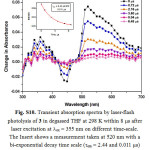 |
Figure S10: Transient absorption spectra by laser-flash photolysis of 3 in degassed THF at 298 K within 8 ms after laser excitation at lex = 355 nm on different time-scale. The Insert shows a measurement taken at 520 nm with a bi-exponential decay time scale (τem = 2.44 and 0.011 μs) and the solid line represents the exponential fitting of the data. Click here to View figure |
Several studies have reported triplet energy transfer from a metal ion center to an aryl hydrocarbon,32–36 but, in some cases, the photosystems were unstable with respect to the sensitized oxygenation of the polycycle36.Meyer and co-workers37 reported an intense transient absorption at 450-600 nm, which is characteristic of a 4,4′-bpy-localized MLCT excited state in Re(I) complexes. Castellano and co-workers38 postulated that one could potentially observe significantly extended lifetimes in metal complexes if the appropriate chromophores are appended as alkynyl ligands. A detailed study by the Keller and Schanze39 also demonstrated that the 3LC states of alkynyl ligands can markedly influence the excited state decay. Given these facts, the TA band of 2 and 3 can not be assigned to the transient absorption of the pure 3MLCT excited states. The lower region observed at 450-700 nm for 2 and 3 might be expected to be due to the longer lived ligand localized states of alkynyl ligands 40–43. Hence the prolonged lifetimes observed for 2 and 3 might be due to the triplet state localized on the Re(I) chromophores being in equilibrium with the triplet states associated with the 3LLCT and 3IL excited states. This prompts us to propose that the difference in the 3MLCT energies of 1-3 may be smaller than the energy difference between 3MLCT states and 3LC states.
Since the distance from Re(I) component to naphthalene/anthracene unit is less than 1 nm the most probable mechanism for energy transfer is Dexter mechanism44. In addition, the presence of short lifetime observed by the time-correlated single photon counting technique excludes the Forster mechanism45 as a candidate for the energy transfer mechanism. However, at this stage it is premature to rule out operation of Förster mechanism here and the system will be further studied in order to understand the mechanism clearly. Therefore, it is tentatively assigned that some portion of the excitation light absorbed by the Re(I) chromophore in 2 and 3 is first transferred to the alkynyl ligand (3LC state) and is subsequently redistributed between the luminescent 3LLCT and 3IL excited states and 3MLCT potential levels (Scheme 1). According to this scenario, in the case of 2 and 3, the overall excitation energy collection process includes an indirect, two step Re → alkynyl ligand energy transfer, which is mediated by a spatially interposed alkynylene unit in a manner such that the 3LLCT and 3IL energy levels act as a reservoir for the excitation energy. Thus, it was observed that the variation of the substituents on the alkynyl ligands enable the fine tuning of the emission energy, which would be exceptionally important in the realization of supramolecular electron- and energy-transfer systems.
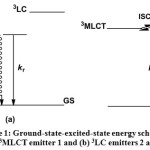 |
Scheme 1: Ground-state-excited-state energy scheme for (a) 3MLCT emitter 1 and (b) 3LC emitters 2 and 3. Click here to View Scheme |
Conclusion
In summary, molecular rectangles are shown, for the first time, to mimic the natural photosynthetic chromophores. Successful emission color tuning is achieved by incorporating rigid alkynyl ligands into 1-3. The blue shift in emission maximum upon extending the p-conjugated system in these complexes represents an unusual phenomenon. Complex 1 which is bridged by a phenyl unit, exhibits a typical strong 3MLCT luminescence behavior while compounds 2 and 3 show IL luminescence through energy transfer process. Thus, the variation of the substituents on the alkynyl ligands enabled the fine tuning of the emission energy of 1-3. Future research along these lines would also involve the integration of these artificial antennae into electron/energy transfer relay systems to achieve artificial photosynthesis.
Acknowledgements
We thank Council of Scientific and Industrial Research (CSIR), New Delhi for financial support in the form of a project. Prof. S.R thanks UGC, New Delhi for sanctioning UGC-BSR Faculty and UGC Emeritus Fellowships. P.T thanks Academia Sinica and the National Science Council of Taiwan for financial support. A.R is the recipient of UGC Meritorious fellowship under the Basic Scientific Research (BSR) Scheme.
References
- Kuhlbrandt, W. Many wheels make light work. Nature, 1995, 374, 497-498.
CrossRef - Hu, X.; Damjanovic, A.; Ritz, T.; Schulten, K. Architecture and mechanism of the light-harvesting apparatus of purple bacteria. Proc. Natl. Acad. Sci. U.S.A., 1998, 95, 5935-5941.
CrossRef - Frischmann, P. D.; Mahata, K.; Wurthner, F. Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem. Soc. Rev., 2013, 42, 1847-1870.
CrossRef - Jalal, K.C.A.; Zaima Azira, Z.A.; Nor Hafizah, Z.; Rahman, M. M.; Kamaruzzaman, B.Y.; Noor Faizul, H. N. Carotenoid contents in anoxygenic phototrophic purple bacteria, marichromatium sp. and rhodopseudomonas sp. of tropical aquatic environment, Malaysia. Orient. J. Chem., 2014, 30, 607-613.
CrossRef - Cooke, M. W.; Hanan, G. S.; Loiseau, F.; Campagna, S.; Watanabe, M.; Tanaka, Y. The structural and functional roles of rhodium(II)–rhodium(II) dimers in multinuclear ruthenium(II) complexes. Angew. Chem. Int. Ed., 2005, 44, 4881-4884.
CrossRef - E. Baranoff, J.-P. Collin, L. Flamigni, J.-P. Sauvage, From ruthenium(II) to iridium(III): 15 years of triads based on bis-terpyridine complexes. Chem. Soc. Rev., 2004, 33, 147-155.
CrossRef - Uthayanila, S.; Neeraja, P. Synthesis, characterization and in-vitro cytotoxic studies of 5,10,15,20- tetra pyridyl porphyrin coordinated to four [Ru (bipy)2Cl]+ groups. Orient. J. Chem., 2015, 31, 867-673.
CrossRef - Splan, K. E.; Massari, A. M.; Morris, G. A.; Sun, S.-S.; Reina, E.; Nguyen, S. T.; Hupp, J. T. Photophysical and energy-transfer properties of (salen)zinc complexes and supramolecular assemblies. Eur. J. Inorg. Chem., 2003, 2348-2351.
CrossRef - Splan, K. E.; Keefe, M. H.; Massari, A. M.; Walters, K. A.; Hupp, J. T. Synthesis, characterization, and preliminary intramolecular energy transfer studies of rigid, emissive, rhenium-linked porphyrin dimers. Inorg. Chem., 2002, 41, 619-621.
CrossRef - Sautter, A.; Kaletas, B. K.; D. G. Schmid, R. Dobrawa, M. Zimine, G. Jung, I. H. M. van Stokkum, L. De Cola, R. M. Williams, F. Wurthner, Ultrafast energy-electron transfer cascade in a multichromophoric light-harvesting molecular square. J. Am. Chem. Soc., 2005, 127, 6719-6729.
CrossRef - Tang, M.-C.; Tsang, D. P.-K.; Wong, Y.-C.; Chan, M.-Y.; Wong, K.M.-C.; Yam, V.W.-W. Bipolar gold(III) complexes for solution-processable organic light-emitting devices with a small efficiency roll-off. J. Am. Chem. Soc., 2014, 136, 17861-17868.
CrossRef - Yu, S.; Zeng, Y.; Chen, J.; Yu, T.; Zhang, X.; Yang, G.; Li, Y. Intramolecular triplet–triplet energy transfer enhanced triplet–triplet annihilation upconversion with a short-lived triplet state platinum(II) terpyridyl acetylide photosensitizer. RSC Adv., 2015, 5, 70640-70648.
CrossRef - Hung, L.-L.; Lam, W. H.; Wong, K.M.-C.; Cheng, E.C.-C.; Zhu, N.; Yam, V.W.-W. Synthesis, luminescence and electrochemical properties of luminescent dinuclear mixed-valence gold complexes with alkynyl bridges. Inorg. Chem. Front., 2015, 2, 453-466.
CrossRef - Chan, A.K.-W.; Wong, K.M.-C.; Yam, V.W.-W. Supramolecular assembly of isocyanorhodium(I) complexes: An interplay of rhodium(I)···rhodium(I) interactions, hydrophobic–hydrophobic interactions, and host–guest chemistry. J. Am. Chem. Soc., 2015, 137, 6920-6931.
CrossRef - Lam, S.-T.; Wang, G.; Yam, V.W.-W. Luminescent metallogels of alkynylrhenium(I) tricarbonyl diimine complexes. Organometallics, 2008, 27, 4545-4548.
CrossRef - Lin, J. T.; Sun, S.-S.; Wu, J .J.; Lee, L.; Lin, K.-J.; Huang, Y. F. Dinuclear metal carbonyls bridged by pyridyl ligands incorporating an alkyne entity. Inorg. Chem. 1995, 34, 2323-2333.
CrossRef - Ciana, L. D.; Haim, A. Synthesis of 1,4-bis(4-pyridyl)butadiyne. J. Heterocycl. Chem., 1984, 21, 607-608.
CrossRef - Champness, N. R.; Khlobystov, A.N.; Majuga, A. G.; Schroder, M.; Zyk, N. V. An improved preparation of 4-ethynylpyridine and its application to the synthesis of linear bipyridyl ligands. Tetrahedron Lett., 1999, 40, 5413-5416.
CrossRef - Rajendran, T.; Manimaran, B.; Liao, R.-T.; Lin, R.-J.; Thanasekaran, P.; Lee, G.-H.; Peng, S.-M.; Liu, Y.-H.; Chang, I.-J.; Rajagopal, S.; Lu, K.-L. Synthesis and photophysical properties of neutral luminescent rhenium-based molecular rectangles. Inorg. Chem., 2003, 42, 6388-6394.
CrossRef - Rajendran, T.; Manimaran, B.; Liao, R.-T.; Liu, Y.-H.; Thanasekaran, P.; Lin, R.-J.; Chang, I.-J.; Chou, P.-T.; Ramaraj, R.; Rajagopal, S.; Lu, K.-L. Luminescence quenching of Re(I) molecular rectangles by quinones. Dalton Trans., 2010, 39, 2928-2935.
CrossRef - Wu, P.-C.; Yu, J.-K.; Song, Y.-H.; Chi, Y.; Chou, P.-T.; Peng, S.-M.; Lee, G.-H. Synthesis and characterization of metal complexes possessing the 5-(2-pyridyl) pyrazolate ligands: The observation of remarkable osmium-induced blue phosphorescence in solution at room temperature. Organometallics, 2003, 22, 4938-4946.
CrossRef - Rajendran, T.; Manimaran, B.; Lee, F.-Y.; Lee, G.-H.; Peng, S.-M.; Wang, C. M.; Lu, K.-L. First light-emitting neutral molecular rectangles. Inorg. Chem. 2000, 39, 2016-2017.
CrossRef - Chung, W.-K.; Wong, K.M.-C.; Lam, W. H.; Zhu, X.; Zhu, N.; Kwok, H.-S.; Yam, V.W.-W. Syntheses, photophysical, electroluminescence and computational studies of rhenium(I) diimine triarylamine-containing alkynyl complexes. New J. Chem. 2013, 37, 1753-1767.
CrossRef - Yam, V.W.-W.; Chong, S.H.-F.; Ko, C.-C.; Cheung, K.-K. Synthesis and luminescence behavior of rhenium(I) triynyl complexes. X-ray crystal structures of [Re(CO)3(tBu2bpy)(C⋮C−C⋮C−C⋮CPh)] and [Re(CO)3(Me2bpy)(C⋮C−C⋮C−C⋮CSiMe3)]. Organometallics, 2000, 19, 5092-5097.
CrossRef - Lam, S.-T.; Zhu, N.; Au, V.K.-M.; Yam, V.W.-W. Synthesis, characterization, electrochemistry and photophysical studies of rhenium(I) tricarbonyl diimine complexes with carboxaldehyde alkynyl ligands. Polyhedron, 2015, 86, 10-16.
CrossRef - Li, M.-J.; Kwok, W.-M.; Lam, W. H.; Tao, C.-H.; Yam, V.W.-W.; Phillips, D. L. Synthesis of coumarin-appended pyridyl tricarbonylrhenium(I) 2,2′-bipyridyl complexes with oligoether spacer and their fluorescence resonance energy transfer studies. Organometallics, 2009, 28, 1620-1630.
CrossRef - Sun, S.-S.; Lees, A. J. Synthesis and photophysical properties of dinuclear organometallic rhenium(I) diimine complexes linked by pyridine-containing macrocyclic phenylacetylene ligands. Organometallics, 2001, 20, 2353-2358.
CrossRef - Li, Z.; Sun, W. Synthesis, photophysics, and reverse saturable absorption of platinum complexes bearing extended π-conjugated C^N^N ligands. Dalton Trans., 2013, 42, 14021-14029.
CrossRef - Winter, A.; Friebe, C.; Chiper, M.; Schubert, U. S.; Presselt, M.; Dietzek, B.; Schmitt, M.; Popp, J. Synthesis, characterization, and electro-optical properties of ZnII complexes with π-conjugated terpyridine ligands. ChemPhysChem, 2009, 10, 787-798.
CrossRef - Grosshenny, V.; Harriman, A.; Romero, F. M.; Ziessel, R. Electron delocalization in ruthenium(II) and osmium(II) 2,2’-bipyridyl complexes formed from ethynyl-bridged ditopic ligands. J. Phys. Chem., 1996, 100, 17472-17484.
CrossRef - Maubert, B.; McClenaghan, N. D.; Indelli, M. T.; Campagna, S. Absorption spectra and photophysical properties of a series of polypyridine ligands containing appended pyrenyl and anthryl chromophores and of their ruthenium(II) and osmium(II) complexes. J. Phys. Chem. A, 2003, 107, 447-455.
CrossRef - El-ghayoury, A.; Harriman, A.; Khatyr, A.; Ziessel, R. Intramolecular triplet energy transfer in metal polypyridine complexes bearing ethynylated aromatic groups. J. Phys. Chem. A, 2000, 104, 1512-1523.
CrossRef - Sathish, V.; Babu, E.; Ramdass, A.; Lu, Z.-Z.; Chang, T. T.; Velayudham, M.; Thanasekaran, P.; Lu, K.-L.; Li, W.-S.; Rajagopal, S. Photoswitchable alkoxy-bridged binuclear rhenium(I) complexes – a potential probe for biomolecules and optical cell imaging. RSC Adv., 2013, 3, 18557-18566.
CrossRef - Barigelletti, F.; Flamigni, L. Photoactive molecular wires based on metal complexes. Chem. Soc. Rev., 2000, 29, 1-12.
CrossRef - El-ghayoury, A.; Harriman, A.; Ziessel, R. Intercompartmental electron exchange in geometrically-constrained Ru−Os triads built around diethynylated aryl hydrocarbons. J. Phys. Chem. A, 2000, 104, 7906-7915.
CrossRef - Belser, P.; Dux, R.; Baak, M.; De Cola, L.; Balzani, V. Electronic energy transfer in a supramolecular species containing the [Ru(bpy)3]2+, [Os(bpy)3]2+, and anthracene chromophoric units. Angew. Chem. Int. Ed. Engl., 1995, 34, 595-598.
CrossRef - Tapolsky, G.; Duesing, R.; Meyer, T. J. Intramolecular energy transfer by an electron/energy transfer cascade. J. Phys. Chem., 1989, 93, 3885-3887.
CrossRef - Hua, F.; Kinayyigit, S.; Rachford, A.A.; Shikhova, E. A.; Goeb, S.; Cable, J. R.; Adams, C. J.; Kirschbaum, K.; Pinkerton, A. A.; Castellano, F. N. Luminescent charge-transfer platinum(II) metallacycle. Inorg. Chem., 2007, 46, 8771-8783.
CrossRef - Keller, J. M.; Schanze, K. S. Synthesis of monodisperse platinum acetylide oligomers end-capped with naphthalene diimide units. Organometallics, 2009, 28, 4210-4216.
CrossRef - Ziessel, R. F. Photo-induced energy or electron transfer in supramolecular systems: Applications to molecular wires and light-harvesting sensors. J. Chem. Edu., 1997, 74, 673-679.
CrossRef - Otsuki, J.; Imai, A.; Sato, K.; Li, D.-M.; Hosoda, M.; Owa, M.; Akasaka, T.; Yoshikawa, I.; Araki, K.; Suenobu, T.; Fukuzumi, S. Switchable Antenna: A star-shaped ruthenium/osmium tetranuclear complex with azobis(bipyridine) bridging ligands. Chem. –Eur. J., 2008, 14, 2709-2718.
CrossRef - Welter, S.; Lafolet, F.; Cecchetto, E.; Vergeer, F.; De Cola, L. Energy transfer by a hopping mechanism in dinuclear IrIII/RuII complexes: A molecular wire?. ChemPhysChem, 2005, 6, 2417-2427.
CrossRef - Ichimura, K.; Kobayashi, T.; King, K. A.; Watts, R. J. Excited-state absorption spectroscopy of ortho-metalated iridium(III) complexes. J. Phys. Chem. 1987, 91, 6104-6106.
CrossRef - Dexter, D. L. A theory of sensitized luminescence in solids. J. Chem. Phys., 1953, 21, 836-850.
CrossRef - Forster, T. 10th spiers memorial lecture. Transfer mechanisms of electronic excitation. Discuss. Faraday Soc., 1959, 27, 7-17.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









