Synthesis and Antimicrobial Activity of Azetidin-2-One Fused 2-Chloro-3-Formyl Quinoline Derivatives
Govind Nayak1*, Birendra Shrivastava1 and Akhlesh Kumar Singhai2
1School of Pharmaceutical Sciences, Jaipur National University, Jaipur- 302 025 (India).
2Lakshmi Narain College of Pharmacy, Bhopal- 462 021 (India).
Corresponding Author E-mail: nayak.govind@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/320423
Azetidin-2-one fused 2-chloro-3-formyl quinolines derivatives, 3-chloro-4-(2-chloro-8/7/6-methoxyquinolin-3-yl)-1-(2,4-dinitro/4-nitro phenylamino)azetidin-2-one,3-chloro-4-(2-chloro-8/7/6-chloroquinolin-3-yl)-1-(2,4-dinitro/4-nitro phenylamino)azetidin-2-one, 3-chloro-4-(2-chloro-8/7/6-methylquinolin-3-yl)-1-(2,4-dinitro/4-nitrophenylamino) azetidin-2-one were synthesized by four steps, respectively from N-arylacetamides, 2-chloro-3-formyl quinolines, 2,4-dinitro/4-nitro phenyl hydrazine reflux with chloroacetyl chloride and triethyl amine. However yields of quinolines having electron donating groups in all cases. The structures of the synthesized compounds have been established on the basis of physical and spectral data. The antibacterial and antifungal activity of these compounds was tested by filter paper disc method against Staphylococcus aureus (MTCC96), Escherichia coli (MTCC722) and Candida albicans (MTCC183). The results showed that azetidin-2-one fused 2-chloro-3-formyl quinolines derivatives are better in inhibiting the growth of both types of organisms. Compounds AZT b2, AZT b3 to AZT g2, AZT g3 were found to be more potent compared to standard drug.
KEYWORDS:2-chloro-3-formyl-quinoline; Vilsmeier-Haack reagent; 2, 4-dinitro/4-nitro phenyl hydrazine; triethyl amine; chloroacetyl chloride; antimicrobial activity
Download this article as:| Copy the following to cite this article: Nayak G, Shrivastava B, Singhai A. K. Synthesis and Antimicrobial Activity of Azetidin-2-One Fused 2-Chloro-3-Formyl Quinoline Derivatives. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Nayak G, Shrivastava B, Singhai A. K. Synthesis and Antimicrobial Activity of Azetidin-2-One Fused 2-Chloro-3-Formyl Quinoline Derivatives. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=18868 |
Introduction
Substituted quinolines are very simply, efficiently and conveniently synthesized in less time and high yield with less amount of raw material under mild condition with the help of Vilsmeier-Haack reagent (DMF+POCl3) and substituted acetanilide. Quinoline has been posssess a wide spectrum of biological activities.Now a days quinoline derivatives are used for a convenient starting material of various further substituted quinoline.
Formylation of heterocyclic compounds1, may be achieved by heating heterocyclic compound with Vilsmeier reagent i.e (DMF+POCl3) phosphorous oxychloride and dimethyl formamide, the intermediate is hydrolysed in the presence of mild base give 2-(ortho)-substituted heterocyclic compound2. This reaction is known as Vilsmeier-Haack reaction. The treatment of infectious diseases still remains an important and challenging problem. The search of novel antimicrobial agents is a field of current and growing interest. Many compounds have been synthesized with this aim; their clinical use has been limited by their relatively high risk of toxicity, bacterial resistance and/or pharmacokinetic deficiencies3-4. According to the literature, pyrazoloquinoline and triazolo-thiadiazole quinoline derivatives are reported to possess various biological activities such as antibacterial5, antifungal6 anti-inflammatory7. As a part of our continuing interest, we have investigated for the first time cyclic azetidin-2-one fused 2-chloro-3-formyl quinoline derivatives with an aryl hydrazine group.
Methods and Materials
All chemicals were used of analytical grade from Chemdyes Corporation (Chemco) limited. All melting points were taken in open capillaries tube and are an correct. The progress of all reaction of the synthesized compound was monitored by TLC i.e (Thin layer chromatography). TLC was run using silica gel PF254, 366 as an adsorbent and ethyl acetate – n-hexane in different ratio as eluent. Spot were visualized using solid fumes, by keeping of TLC plate in iodine chamber.
Reaction
Experimental Procedure for
IR spectra were recorded on a FT-IR (Bruker) spectrophotometer, 1HNMR Bruker Avance II 400 MHz instrument using DMSO as solvent and TMS as internal reference (Chemical shift in δ, ppm). The following abbreviations were used to indicate the position of functional groups in term of stretching and bending (FT-IR), peak multiplicity s-singlet, d-doublet, t-triplet, q-quartet, m-multiplet, dd-doublet of doublet (1HNMR).
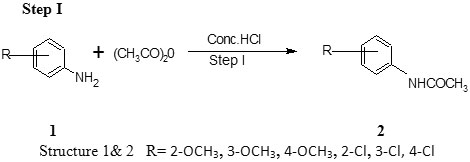
Step I Preparation of ortho substituted acetanilide8, 9
Aniline (5 ml) is dissolved in hydrochloric acid (4.6 ml concentrated hydrochloric acid and 12.5ml water) in a beaker. To the clear solution are added acetic anhydride (6.5 ml).The mixture is stirred until acetic anhydride has completely reacted. The mixture are immediately poured in to a solution of sodium acetate (8.3 gm) in water (25 ml).The solution is stirred and cooled in ice. The separated acetanilide is filtered. It is recrystallized from boiling water (100-125 ml) to which ethyl alcohol has been added (Table 1).
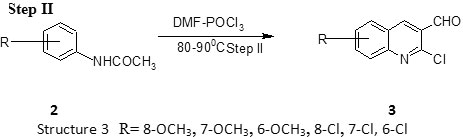
Step II Preparation of 2-chloro-3-formyl-quinoline CFQ 8, 9
To a solution of acetanilide (N-phenylacetamide) (5 mmoles) in dry DMF (15 mmoles) at 0-5oC POcl3 (60 mmoles) was added dropwise
with stirring and the mixture was then stirred at 80 – 100oC for time ranging between 4-16 hr. The mixture was poured on to crush ice, stirred for 5 minutes and the resulting solid filtered, washed well with water and dried. The compounds were recrystallized from ethyl acetate. Phosphoryl chloride (commonly called phosphorus oxychloride) is a colorless liquid with the formula POCl3. It hydrolyses in moist air to phosphoric acid to release choking fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phospha (Table 1).
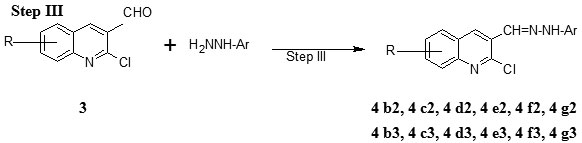
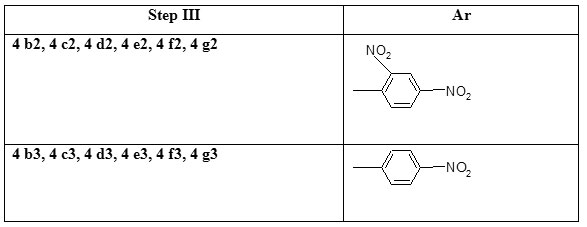
Step III Preparation of 2-chloro-8-methoxy-3-(2, 4-dinitro/4-nitro phenylhydrazono) methyl) quinoline (4b2-4g2 and 4 b3-4 g3)
To a DMF solution of 2-chloro-8-methoxyquinoline-3-carbaldehyde 6 mmoles were added aryl hydrazine (phenyl hydrazine 11 mmoles) and refluxed for three hours, and then left to cool to room temperature or the solvent was removed and the separated solid was poured in to the water. The precipitated product was filtered, washed well with water and dried (Table 1).
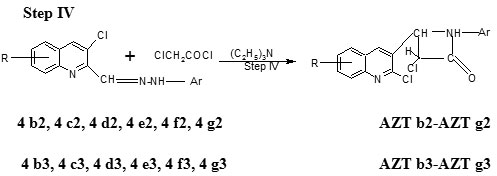
Step IV Preparation of 3-chloro–4-(2-chloro–8-methoxyquinolin–3-yl) – 1- (2, 4-dinitro/4-nitro phenylamino)–azetidin–2-one (AZT b2-AZTg2 and AZT b3-AZT g3)
The compound 2-chloro-8-methoxy-3-((2, 4-dinitro/4-nitro phenylhydrazono) methyl) quinoline step-III b1 (0.01 mol) was dissolved in DMF (40ml) and triethylamine (0.02 mol) was added to it. Chloroacetyl chloride (0.02 mol) was added dropwise a period of 30 minutes. The reaction mixture was refluxed for 5 hr, and filtered to separate the solid formed. The filtrate was poured on to crushed ice, the product was filtered and recrystallized from ethylacetate (Table 1).
Characterization data of the synthesized compound10-11
Acetanilide
FT-IR cm-1 3295 (N-H), 1664 (CO), 1584 (C=C), 1HNMR (400 MHz, DMSO), δ; 8.72 (s, 1H, NH), 2.1 (s, 3H, CH3), 7.2 (d, 1H, Ar-H).
2-Chloro-3-formyl quinoline (CFQ)
FT-IR cm-1 2896.53 (C – H Str,Ar) 1684.39 (C=O Str) 1574.14 (C=N Str) 1455.03 (C = C Str) 1367.35 (C – N Str,Ar) 749.25 (C – Cl Bending) 1HNMR (400 MHz, DMSO), δ; 10.5 (s, 1H, CHO), 8.8 (s, 1H, H-4), 8.1 (m, 1H, H-6), 7.7 (m, 1H, H-7)
3-chloro–4-(2-chloro–8/7/6-methoxyquinolin–3-yl)-1-(2, 4-dinitro phenylamino) – azetidin – 2 –one (AZT b2, AZT c2, AZT d2)
FT-IR cm-1 2998.1(C-H Ar), 3449.10 (N-H Str), 1682.51 (C=O Str), 1139.46 C – N (Str Ar), 758.25 (C – Cl Bending). 1HNMR (400 MHz, DMSO), δ; 12.10 (s, NH) 9.21 (s, 1H, CHO) 7.98 (s, 1H, C4-H) 7.67 (m,CH-5) 3.83 (s, CH3).
3-chloro–4-(2-chloro–8/7/6-methoxyquinolin–3-yl)-1-(4-nitro phenylamino) – azetidin – 2 – one (AZT b3, AZT c3, AZT d3)
FT-IR cm-1 3262.86 (N– H Str, Ar) 1696.94 (C=O Str) 1566.45 (C=N Str) 1592.64 (N-H bending in amide) 1052.68 (CH3O Str). 1HNMR (400 MHz, DMSO), δ; 7.82 – 8.01 (m, Ar-H) 10.37 (s, 1H, CH heteroaromatic proton) 8.43 (s, 1H, H-4) 7.79 (d, C=O) 4.1 (d, 1H CH-Cl ) 3.35 (s, 3H, OCH3 ) 7.32 (d, 1H, H-5).
3-chloro-4-(2,8/7/6-dichloroquinolin-3-yl)-1-(2, 4-dinitro phenylamino) azetidin-2-one (AZT e2, AZT f2, AZT g2)
FT-IR cm-1 3487.00 (C – H Str, Ar) 3306.34 (N-H Str) 1748.36 (C=O Str) 1519.21 (C = N Str) 1335.03 (C – N Str, Ar) 1060.31 (CH3O, Str) 794.11 (C – Cl, Str) 722.06 (C – Cl Bending). 1HNMR (400 MHz, DMSO), δ; 7.31 – 8.17 (m,Ar-H) 10.16 (s, 1H, CH Heteroaromatic proton) 8.78 (s, 1H, H-4) 8.34 (m, 1H, H-8) 7.28 (C=O group d, 1H) 3.17 (m, CH-N aromatic)
3-chloro-4-(2, 8/7/6-dichloroquinolin-3-yl)-1-(4-nitro phenylamino) azetidin-2-one (AZT e3, AZT f3, AZT g3)
FT-IR cm-1 3362.55 (C – H Str, Ar) 1681.83 (C=O Str) 1531.60 (C = N Str) 1418.67 (C – N Str, Ar) 776.88 (C – Cl, Bending).1HNMR (400 MHz, DMSO), δ; 7.40 – 8.01 (m,Ar-H) 10.24 (s, 1H, CH Heteroaromatic proton) 8.03 (s, 1H, H-4) 7.50 (m, 1H, H-7) 6.58 (C=O, d, 1H).
Table 1: Physical data of synthesized compounds
|
S. No. |
Code |
Molecular formula |
Molecular Weight (gm) |
Melting point |
Percentage yield |
Rf value (Ethylacetate: n-hexane) |
|
1. |
CFQ |
C10H6NOCl |
191.616 |
144-146oC |
79 |
0.67 |
|
2. |
AZT b2 |
C19H13Cl2N5O6 |
478.690 |
264-267oC |
74 |
0.76 |
|
3. |
AZT b3 |
C19H14Cl2N4O4 |
434.231 |
210-214oC |
55 |
0.65 |
|
4. |
AZT c2 |
C19H13Cl2N5O6 |
478.690 |
228-235oC |
68 |
0.69 |
|
5. |
AZT c3 |
C19H14Cl2N4O4 |
434.231 |
186-189oC |
61 |
0.89 |
|
6. |
AZT d2 |
C19H13Cl2N5O6 |
478.690 |
219-225oC |
81 |
0.58 |
|
7. |
AZT d3 |
C19H14Cl2N4O4 |
434.231 |
164-167oC |
74 |
0.68 |
|
8 |
AZT e2 |
C18H10Cl3N5O5 |
482.321 |
211-214oC |
80 |
0.89 |
|
9 |
AZT e3 |
C18H11Cl3N4O3 |
436.542 |
189-192oC |
89 |
0.75 |
|
10 |
AZT f2 |
C18H10Cl3N5O5 |
482.321 |
196-198oC |
84 |
0.72 |
|
11 |
AZT f3 |
C18H11Cl3N4O3 |
436.542 |
167-169oC |
64 |
0.61 |
|
12 |
AZT g2 |
C18H10Cl3N5O5 |
482.321 |
187-192oC |
56 |
0.97 |
|
13 |
AZT g3 |
C18H11Cl3N4O3 |
436.542 |
156-162oC |
92 |
0.79 |
Antimicrobial activity12-18
The compounds AZT b2 – AZT g3 were tested for their antibacterial activity and antifungal activity by filter paper disc method using nutrient broth medium (contained g/L: beef extract 30gm; Casein hydrolysate 17.5 gm; soluble starch 1.5gm; pH 7.4). The Gram-positive and Gram-negative bacteria utilized in this study consisted of Staphylococcus aureus, Escherichia coli and fungi Candida albicans. In the filter paper disc method, sterile paper discs (05mm) impregnated with compound dissolved in DMF (Dimethyl formamide) at concentration 200µg/mL were used. Then, the paper disc impregnated with the solution of the compound tested was placed on the surface of the media inoculated with the microorganism. The plates were incubated at 37oC for sufficient period of time. After incubation were noted and the results are given in Table 2.
Table 2: Antimicrobial activities of Compounds AZT b2-AZT g3 (Diameter of the zone of inhibition in mm)
|
Compound |
Antibacterial activity |
Antifungal activity |
|
|
Staphylococcus aureus (MTCC96) |
Escherichia coli (MTCC722) |
Candida albicans (MTCC183) |
|
|
AZT b2 |
11 |
9 |
10 |
|
AZT b3 |
14 |
13 |
8 |
|
AZT c2 |
13 |
14 |
11 |
|
AZT c3 |
15 |
13 |
9 |
|
AZT d2 |
11 |
8 |
9 |
|
AZT d3 |
14 |
14 |
12 |
|
AZT e2 |
13 |
13 |
10 |
|
AZT e3 |
15 |
12 |
9 |
|
AZT f2 |
11 |
6 |
7 |
|
AZT f3 |
14 |
13 |
11 |
|
AZT g2 |
13 |
14 |
8 |
|
AZT g3 |
15 |
12 |
10 |
|
Standard |
18 |
18 |
13 |
|
*Control |
0 |
0 |
0 |
* The solvent (control) is Dimethy formamide
1. Staphylococcus aureus reference compound Amikacin
2. Escherichia coli reference compound Amikacin
3. Candida albicans reference compound Ketoconazole
Results and Discussion
All the synthesized compounds were confirmed by their spectral data (Table 1) and the screened for anti-bacterial against Staphylococcus aureus, Escherichia coli and ant-fungal activity against Candida albicans, compounds (AZT b2-AZT g2) showed moderate to good antibacterial and antfungal activity (Table 2) when compared to that of reference Amikacin and Ketoconazole respectively. The structures of all compounds were confirmed by FT-IR and 1H NMR spectra (2.3 sections). The FT-IR spectra of the azetidine fused 2-chloro-3-formyl quinoline derivatives AZT b2 –AZT g2 showed absorption bands at about 1748-1708 cm-1 characteristic for C=O stretching vibration, 1528-1519 cm-1 for C=N Stretching associated with quinoline 2927 cm-1 for C-H aromatic stretching, 759 cm-1 absorption for C-Cl stretching, absorption band at 3306.34 cm-1 for N-N=C vibration provided confirmatory evidence for ring closure. Further support was obtained from the 1HNMR spectra, resonance assigned 10.6 δ (s, 1H, CHO), 8.5 δ (s,1H, H-4), 2.6 δ (s, 3H, CH3) for 6-methyl/7-methyl/8-methyl (2.8 δ, s,3H), 4.0 δ (s, 3H, OCH3) for 6-methoxy/7-methoxy/8-methoxy, 10.7 (s, 1H, CHO), 8.5 (s, 1H, H-4), 7.7 (m, 1H, H-5), 7.5 (s, 1H, H-8), 7.2( m, 1H, H-6), 10.8 (s, 1H, CHO), 8.6 (s, 1H, H-4), 8.1 (m, 1H, H-8), 7.7 (m, 1H, H-7), 7.6 (s, 1H, H-5) for the confirmation of the compounds. Having obtained chloro and formyl group substituted quinolines the possible transformations of these functionalities could afford the new quinolines (AZT b2-AZT g2), which are equally important synthon for the synthesis of fused quinoline systems.
Acknowledgements
The authors are grateful to the LNCP, Bhopal for providing the necessary facilities to carry out this research work, and also thankful to the SAIF Lab, Panjab university, Chandigarh for recording the spectral data of the compounds.
References
- Meth, C.O.; Narine B. tetrahedron let., 1978,19, 2045-2048.
- Meth, C.O.; Narine B.; Tarnowski B. J. Chem. Soc; perkin Trans-1, 1981, 1520-1530.
- Pett, K. R.; Cage D. G.; Liebman A. J. Int. J. Antimicrobial Agents, 2000, 15, 299.
CrossRef - Savage, P. B. Eur. J. Org. Chem., 2002, 5, 759.
CrossRef - Hirosato, K.; Fumino S.; Kiyotaka K.; Goro T. J. Med. Chem., 1998, 31, 221.
- Pramilla, S. Indian J. Heterocyclic Chem., 1998, 10, 13.
- Gupta, R. K.; Gupta A.; Kachroo P. L. Indian J. Chem., 1998, 37B, 1211.
- Srivastava, A.; Singh R. M. Indian J.Chem., 2005, 44B, 1868-1875.
- John, R. D.; Applications of Absorption Spectroscopy of Organic Compounds, Prentice Hall of India (P): New Delhi 1969, 33-38.
- Silverstein, R.M.; Webster F. X.; Spectrometric Identification of Organic Compounds, John Wiley and Sons: Inc.1998.
- McLafferty, F. W.; Interpretation of Mass Spectra, W.A. Benjamin Inc. publishers: New York 1974.
- Holt, J.G.; Krieg N.R.; Sneath, P.H.; Stanley, J.T.; Williams, S.T.; Bergy’s Manual of Determinative Bacteriology, Williams and Wilkins: Baltimore 1996, 324.
- Chung, K.C.; Goepfert J.M. J. Food. Sci., 1970, 35, 326-328.
CrossRef - Ferreira, M.A.; Lund, B.M. Appl. Microbiology, 1987, 5, 67-70.
CrossRef - Glynn, H. J.; Heinke G.W.; Environmental Science and Engineering Microbiology and Epidemiology, Prentice Hall International: New Jersey 1996, 266.
- Pelczar, M. J.; Chan E.C.S.; Krieg N.R.; Microbiology, McGraw-Hill Book Company: New York 1986, 333, 435.
- Rex, J.H.; Walsh T.J.; Sobel J.D. Clin. Infect. Dis., 2000, 30, 662-678.
CrossRef - Pfaller, M. A.; Diekema, D. J.; Jones R. N. J. Clin. Microbiology, 2001, 39, 3254 – 3259.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









