Studies on The Synthesis of Fluorinated Schiff Bases; Biological Activity of Resulting (E)-N-Benzylidene-2,3,5,6-Tetrafluoropyridin-4-Amine and 4-Amino-2-Ethoxy-3,5,6-Trifluoropyridine
Imane Raache, Lakhdar Sekhri* and Ahmed Tabchouche
Process Engineering Department, Faculty of Applied Sciences, University Kasdi Merbah, Ouargla 30000, Algeria.
Corresponding Author E-mail: sekhril@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/320408
Article Received on :
Article Accepted on :
Article Published : 27 Jul 2016
The present work is aimed mainly to synthesize new fluorinated compounds and their biological significance against tested bacteria and fungus. Thus, the 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 was synthesized by the reaction of 4-amino 2,3,5,6-tetrafluoropyridine 1 with benzaldehyde 2 in EtOH to obtain the pure product as white crystals in 33% yield. The purity of this compound was estimated by TLC technique and microanalysis while its structure was supported by the usual spectroscopic methods such as UV, infrared, 1H. NMR, and gas chromatography coupled by a mass spectrometry (GC/MS). 4-amino 2,3,5,6-tetrafluoropyridine 1 coupled readily to benzaldehyde 2 when we have used THF as solvent resulting a new Schiff base (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 in 48 % yield. Exploration of the anti-bacterial activity against both gram-positive and gram-negative bacteria showed that these compounds 3 and 4 compounds at a concentration of 250 µg/ml exhibited a slight activity towards Enterococcus feacium; Streptocoque B and Staphylococus aureus exhibited a mild/moderate activity for the case of Escherichia coli and Salmonella typhimurium when compared to Ampicillin. From the results obtained by the antifungal activity, it is found that this fluorinated Schiff base 3 and fluorinated ethoxypyridine 4 are more active against Candida albicans at a concentration of 250 µg/ml when compared to Nystatine.
KEYWORDS:synthesis; anti-bacterial activities; antifungal activity; fluorinated Schiff bases
Download this article as:| Copy the following to cite this article: Raache I, Sekhri L, Tabchouche A. Studies on The Synthesis of Fluorinated Schiff Bases; Biological Activity of Resulting (E)-N-Benzylidene-2,3,5,6-Tetrafluoropyridin-4-Amine and 4-Amino-2-Ethoxy-3,5,6-Trifluoropyridine. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Raache I, Sekhri L, Tabchouche A. Studies on The Synthesis of Fluorinated Schiff Bases; Biological Activity of Resulting (E)-N-Benzylidene-2,3,5,6-Tetrafluoropyridin-4-Amine and 4-Amino-2-Ethoxy-3,5,6-Trifluoropyridine. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=19671 |
Introduction
Fluorinated compounds have gained an increasing interest due to their successful applications in different fields of chemistry, such as supramolecular studies; design of new materials and as important starting materials in the syntheses of new molecules with biological functions.1-4
Due to its small steric size, fluorine has been used as a replacement for hydrogen in many biologically active molecules, including amino acids.4 And the introduction of fluorine into an organic molecule via electrophilic or nucleophilic reactions and most commonly, from fluorinated building blocks, have become a key in the search and discovery of new drugs or increasing the activity of those that already exist; fluorine can cause significant changes in an organic molecule, mainly by its electron-withdrawing inductive effect, which in turn causes differences in interactions with biological receptors or enzymes, as well as on the metabolic fate of the drug.5, 6 It is curious that notwithstanding the abundance of this element, natural fluorinated compounds are very rare. In fact, most of the known fluorinated organic compounds have been synthesized in the laboratory.7
On the other hand, Schiff bases, named after Hugo Schiff,8 are formed when any primary amine reacts with an aldehyde or a ketone under specific conditions. Structurally, a Schiff base (also known as imine or azomethine) is an interesting compound with a functional group that contains a carbon–nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group (R2R3C=N-R1). The chain on the nitrogen makes the Schiff bases a stable imine.9, 10
Schiff bases appear to be important intermediates in a number of enzymatic reaction involving interaction of the amino group of an enzyme, usually that of a lysine residue, with a carbonyl group of the substrate.11
In general, Schiff bases have a wide range of potential applications in various biological fields,12 also fluorinated Schiff bases derivatives have been widely studied because they have antifungal, antibacterial,1, 13, 14 DNA cleavage15 and anticancer16 activities which give it attracted remarkable attention.
Thus, in this opportunity we report here the synthesis and characterization of new fluorinated compounds from 4-amino-2,3,5,6-tetrafluoropyridine 1 with benzaldehyde 2. The structural analysis alongside with their preliminary studies of their biological activity on gram-positive and gram-negative bacteria and on Candida Albicans fungus of these compounds is also informed.
Experimental
Material
2,3,4,5,6-Pntafluoropyridine, KOH, and benzaldehyde are used without further purification as well as solvents dichloromethane, ethanol were purchased from Aldrich.
The microbiological material consists of five bacterial pathogens strains: Escherichia coli ATCC 8739 G (-),Salmonella typhimurium ATCC 14028 G (-) Staphylococus aureus ATCC 6538 G (+),Enterococcus feacium ATCC 19434 G (+), Streptocoque B G (+) and a fungus specie is Candida Albicans ATCC 10231; were obtained from INRAP (National Institute of Research and physico-chemical analysis) of Tunisia.
Instrumentation
The FT-IR spectra were performed in the range from 4000 to 400 cm-1on a SHIMADZU 2000 with KBr pellets. Melting points were determined in a Gallenhamp capillary melting points apparatus and were reported without correction. The 1H NMR spectra of 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 and (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 were recorded on a BRUKER Ultrashield Plus 500 spectrometer with frequency of 500 MHz. using DMSO-d6 as solvent and internal standard at room temperature number of scan is (16-1K). Nuclear magnetic resonance (NMR) spectra of 4-amino-2,3,5,6-tetrafluoropyridine 1 were normally recorded at 35°C using a Perkin Elmer R10 or R12 or Perkin Elmer Hitachi R20A spectrometer operating at 60°C MHz for 1H NMR spectra and 54.6 MHz for 19F NMR spectra. Tetramethylsilane (TMS) was used as a reference for 1H NMR spectra and for 19F NMR spectra, chemical shifts were measured relative to trifluoroacetic acid (TFA) as an external interchange reference unless otherwise stated. Positive chemical shifts are in ppm downfield of appropriate reference.
Mass spectra was performed at the INRAP (National Institute of Research and physico-chemical analysis) of Tunisia, The gas chromatograph used is an Agilent 6890, coupled to a mass spectrometer type Agilent 5975B with a quadrupole ionization voltage of 70 eV. The column used is a TR-FAME; with a length of 60 m and an internal diameter equal to 250 µm. The wire thickness being 0.25 μm.
The operating conditions are:
– The temperature of the injector (split mode): 240°C.
– The temperature programming: from 40°C to 260°C at a rate of 2°C/minutes.
– The vector gas used is helium with a flow rate of 0.8 ml / minutes.
Procedure
Preparation of 4-amino-2,3,5,6-tetrafluoropyridine 1
4-amino-2,3,5,6-tetrafluoropyridine 1 was synthesized from 2,3,4,5,6-Pentafluoropyridine (25 g, 148 mmol) was dissolved in THF (175 ml) in a round-bottomed flask equipped with a reflux condenser to give a clear solution. On addition of aqueous ammonia (0.88, 125 ml) a cloudy solution was produced and an exothermic reaction ensued. The mixture was then refluxed for 18 hours. The clear solution produced was poured into water (500 ml) and the whole mixture was extracted with ether (3x75ml). The extract was dried (MgSO4), evaporated using rotary evaporator and the residue freed from the last traces of solvent in vacuo, to give a pale cream solid. Recrystallization of the crude material from light petroleum gave long white needles of 4-amino-2,3,5,6-tetrafluoropyridine 1 (20 g, 0.12 mol, 80%), mp. 85–87°C; IR (KBr, cm-1): 3500-3300 ν(NH str.) 1450-1190 νPy-F; 1H (CDCl3): δ 5.05 (2H, br s, -NH2; 19F (CDCl3): δ 15.1 (2F, m, F-2 and F-6), -85.1 (2F, F-3 and F-5); MS: m/z 166 ([M]+,100%).
Preparation of 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4
0.93 g (5 mmol) of 4-amino-2,3,5,6-tetrafluoropyridine 1 was added to potassium hydroxide 0.28 g (5 mmol) in 15 ml of ethanol contained in 50 mL three-necked round bottom flask equipped with a reflux condenser maintained under nitrogen. The mixture was stirred and heated to reflux for 1h. Then 0,53g (5 mmol) benzaldehyde was then added and the mixture was stirred at room temperature for 72 hours. The reaction was followed by TLC (petroleum ether /acetone, 95:5%). After filtration, the filtrate was vaporated using rotary evaporator and the residue freed from the last traces of solvent in vacuo, to afford a pale white solid which was then recrystallized from ethanol to yield a white microcrystalline powder corresponding to 2-ethoxy-3,5,6-trifluoropyridin-4-amine (0.66g, mmol, 48.52%), mp. 80–83°C; The product was identified by comparison its spectra with those of an authentic sample: IR (KBr,cm-1): 3500-3300 ν(NH str.) 1450-1190 νPy-F, 1250 ν(C-O asym str.) and 1040 ν(C-O sym str.); 1H NMR (500 MHz, DMSO d6): δ 1.4 (3H, t, CH3), 4.2 (2H, q, CH2) and 6.7 (2H, br s, -NH2; MS: m/z 192 ([M]+, 66.6%), 177 ([M-Me]+, 29.33%),164 ([MH-Et]+,100%).
Preparation of (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3
The previous procedure was used except THF was used instead of Ethanol. 0.83g of (5 mmol) of 4-amino-2,3,5,6-tetrafluoropyridine 1 was added to potassium hydroxide 0.28g (5 mmol) in 15 ml of ethanol contained in 50 mL three-necked round bottom flask equipped with a reflux condenser maintained under nitrogen. The mixture was stirred and heated to reflux for 1h. Then 0.53 g (5 mmol) benzaldehyde was then added and the mixture was stirred at room temperature for 72h. The reaction was followed by TLC (petroleum ether /acetone, 95:5%). After evaporation under vacuum a cream powder was obtained corresponding to new compound 3 (0.66 g, 48.52%), mp. 80–83°C; IR (KBr, cm-1): 3000–2850 ν C-H Aromatic, 1660–1614 νC=N, 1100 νPy-F; MS: m/z 254 ([M]+,30.7%), 91(C7H7]+, 100%).
In the FT-IR spectra of the imino-compound 3, a stretching vibration bands due to the presence of the C-H aromatic were observed in the range of 3000–2850 cm-1. However, more important resulted the observation of strong stretching vibration bands due to the presence of the imino functional group (C=N) in the range of 1660–1614 cm-1. The strong bands around 1100 cm-1 are attributed to the C-F stretching vibrations.
Antimicrobial studies
The evaluation of the antibacterial and Antifungal effects was tested by the agar diffusion test according to R. Alizadeh et al.15
Antibacterial activity
The synthesized Schiff base (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 and 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 were screened for their in vitro antibacterial activity against Gram–negative and Gram-positive bacterial strains.
A suspension of the tested microorganisms was spread on the appropriate solid media plates and incubated overnight at 37°C. After 1 day, 4-5 loops of pure colonies were transferred to saline solution in a test tube for each bacterial strain and adjusted to the 0.5Mc Farland turbidity standards (~108 cells/mL). Sterile cotton dipped into the bacterial suspension and the agar plates were streaked three times, each time turning the plate at a 60° angle and finally rubbing the swab through the edge of the plate. Sterile paper discs (Glass Microfibre filters, Whatman; 6mm in diameter) were placed onto inoculated plates and impregnated with different concentrations of the Schiff base. Ampicillin (10 μg/disc) and solvent (DMSO) were used as positive and negative control for all strains.
Inoculated plates with discs were placed in a 37°C incubator. After 24 hours of incubation, the results were recorded by measuring the zones of growth inhibition surrounding the disc. Clear inhibition zones around the discs indicated the presence of antimicrobial activity. The test was run in duplicate.
Antifungal activity
All cultures were routinely maintained on Sabouraud Dextrose Agar (SDA) and incubated at 28°C. The inoculums of non–sporing fungi, Candida albicans were performed by growing the culture in Sabouraud Dextrose broth at 37°C for overnight. Volume of 0.1 ml of diluted fungal culture suspension was spread with the help of spreader on SDA plates uniformly. Sterile 6 mm discs were impregnated with the test complexes. Wells of 6 mm size were cut and loaded with different concentrations of the Schiff base. Antibiotic disc, Nystatin (100 µg/disc) was used as positive control C. albicans plates were incubated at 37 °C for 18−48 h and antifungal activity was determined by measuring the diameters of the inhibition zone (mm).
Results and Discussion
1-Synthesis part
The strategy we have adopted for the synthesis of fluorinated Schiff base, (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 and fluorinated ethoxy aminopyridine 4 was to condense 4-amino-2,3,5,6-tetrafluoropyridine 1 with benzaldehyde 2 by using EtOH as solvent and again by using THF as shown in (Scheme 1). The analytical and physical data are listed in Table 1.
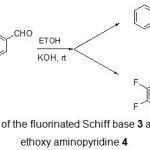 |
Scheme 1: Synthesis of the fluorinated Schiff base 3 and fluorinated ethoxy ethoxy aminopyridine 4 |
Table 1: Analytical data for compounds 1, 3 and 4
| Compounds | Molecular Formula | Melting Point °C | Yield (%) | Physical state |
| 1 | C5H2N2F4 | 85-87 | 80 | long white needles |
| 34 | C7H4N2F4C7H4N2OF3 | 80-8380-83 | 48,5248,52 | white microcrystalline powder |
(E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3
As we might expect, the fluorinated base schiff 3 was successefully obtained when we used THF, and its structure was confirmed by the following spectroscopic data.
FT-IR spectrometry analysis
In the FT-IR spectra of the imino-compound 3, a stretching vibration bands due to the presence of the C-H aromatic were observed in the range of 3000–2850 cm-1. However, more important results were the observation of strong stretching vibration bands due to the presence of the imino functional group (C=N) in the range of 1660–1614 cm-1. The strong bands around 1100 cm-1 are attributed to the C-F stretching vibrations.
Gas chromatography–mass spectrometry GC-MS analysis
Analytical GC-MS (70 eV) (Gas Chromatography-Mass Spectrometry) of the Schiff base revealed the presence of one component only as shown in (Fig.1).
 |
Figure 1: GC-MS Chromatogram of (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 Click here to View Figure |
Mass spectroscopy
The mass spectrum was characteristic of a schiff base with a molecular ion at m/z 254 (66.6%); the major breakdown pattern is tbenzyl (C6H5CH2 ion at m/z192 (100%) (Fig. 2).
 |
Figure 2: Mass spectra of (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 Click here to View Figure |
4-amino-2-ethoxy-3,5,6-trifluoropyridine 4
However, when we used EtOH as solvent, only 4 was produced and no schiff base 3 obtained. This coud be interpreted that the presence of EtO-/EtOH in the mixture favorized the nucleophilic substitution in order to yield 4 instead the nucleophilic addition to the carbonyl group of aldehyde in order to give the schiff base 3.
In 2005 we reported that the reaction of 2,3,5,6-tetrafluoro-4-(2,4,6-trimethylphenylazo)pyridine with an equimolar of sodium methoxide in methanol gave 2,3,5-trifluoro-6-methoxy-4-(2,4,6-trimethylphenylazo) pyridine.17 This reaction is analogous to a reaction we carried out in this project which involved treating 4-amino 2,3,5,6-tetrafluoropyridine 1 with equimolar of EtO-/EtOH. Moreover, the formation of product 4 provides further evidence that nucleophilic substitution in amino-compound 1 by Nu (KOEt) is directed strongly to the ortho positions.
As we might expected, the amino-compound 1 requires no more than its own inbuilt capicity for electron withdrawal and is itself attacked by powerful nucleophiles, e.g., by –OEt (sodium OEt ethoxide) in methanol. The F- leaving group is helped off by EtOH, HF being evolved and –OEt regenerated (Scheme-2).
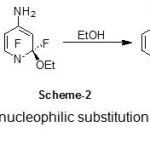 |
Scheme 2: Mechanism of nucleophilic substitution in amino-compound 1 by Nu (KOEt) |
The structure of compound 4 was confirmed by the following spectroscopic data:
FT-IR spectrometry analysis
The IR spectrum showed that the amino group was still present (3500-3300 ν(NH str.), 1450-1190 νPy-F, 1250 ν(C-O asym str.) and 1040 ν(C-O sym str.)
Gas chromatography–mass spectrometry analysis
Analytical GC-MS (70 eV) (Gas Chromatography-Mass Spectrometry) of 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 revealed the presence of one component only as shown in (Fig. 3).
 |
Figure 3: GC-MS Chromatogram of amino-2-ethoxy-3,5,6-trifluoropyridine 4 Click here to View Figure |
Mass spectroscopy
The mass spectrum showed a molecular ion at m/z 192 and base peak at m/z 164 as shown in (Fig. 4).
 |
Figure 4: Mass spectra of amino-2-ethoxy-3,5,6-trifluoropyridine 4 Click here to View Figure |
1H NMR spectrometry analysis
1H NMR spectrum comprises three absorptions of intensity 3: 2 : 2 at 1.4 (3H, t, CH3), 4.2 (2H, q, CH2) and 6.7 (2H, br s, -NH2) as shown in (Fig.5).
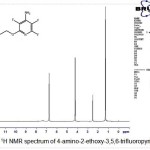 |
Figure 5: 1H NMR spectrum of 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 |
Starting material 4-amino-2,3,5,6-tetrafluoropyridine 1
The purity of starting material 1 was confirmed by spectroscopic data:
Analytical GC-MS (70 eV) (Gas Chromatography-Mass Spectrometry revealed the presence of one component only as shown in (Fig. 6).
; the FT-IR showed that a free amine group is present and this was confirmed by the down-field shift in the N-H stretching and bending bands around 3500–3300 cm-1. The strong bands around 1100 cm-1 are attributed to the C-F stretching vibrations. 1H NMR showed the chemical shift δ 6.7 and this due to the presence of –NH2, Whereas the chemical shifts δ 15.1 and -85.1 of 19F NMR are attributed respectively to the (F-2 and F-6), (F-3 and F-5); mass spectrum complied with a normal fragmentation mechanism for this compound: m/z: 166 ([M]+,100%) as shown in (Fig. 7).
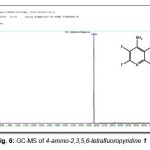 |
Figure 6: GC-MS of 4-amino-2,3,5,6-tetrafluoropyridine 1 |
 |
Figure 7: Mass spectra of 4-amino-2,3,5,6-tetrafluoropyridine 1 |
Antibacterial activity part
4-amino-2-ethoxy-3,5,6-trifluoropyridine 4
Antibacterial Activity
The antibacterial activity of the 2-ethoxy-3,5,6-trifluoropyridin-4-amine 4 was evaluated by the agar diffusion method at concentrations of 100; 150; 200; 250 µg/ml using Dimethyl Sulfoxide (DMSO) as solvent and ampicillin as standard drug. Inhibition areas were measured in mm at the end of 24 h of incubation at 35 °C. The results are reported in Table 2 and figure 8.
From the screening results, it is clearly observed that the fluorinated compound 4 showed a significant activity towards Escherichia coli; Streptocoque B and Salmonella typhimurium at concentration 200; 250 and 300 µg/ml, a mild/moderate activity for the case of Staphylococus aureus; Streptocoque B and Enterococcus feacium at concentration 100; 150; 200 µg/ml when compared to Ampicilline.
Table 2: Antibacterial activity of 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4
| diameters of the inhibition (mm) | ||||||
| Concentration (µg/ml) | Ampicilline10µg | 100 | 150 | 200 | 250 | 300 |
| Escherichia coliATCC 8739 G(-) | 12±1.4 | 8,75±0.3 | 10,25±0.3 | 11±0.7 | 12,75±1.0 | 14±1.4 |
| Salmonella typhimuriumATCC 14028 G(-) | 14,5±0.7 | 9±0.0 | 10,75±0.3 | 11,5±0.7 | 13,5±0.7 | 14,5±0.0 |
| Staphylococus aureusATCC 6538 G(+) | 31,5±0.7 | 9.5±0.7 | 10,5±0.0 | 11,25±0.3 | 13±0.0 | 14.25±0.3 |
| Enterococcus feaciumATCC 19434 G(+) | 37.75±0.3 | 8,5±0.0 | 10,5±0.7 | 13,5±0.7 | 14,75±0.3 | 16,5±0.7 |
| Streptocoque B(Streptococcus agalactiae) G(+) | 26,5±0.7 | 9,25±0.3 | 10,25±1.7 | 11,75±2.4 | 14,5±0.7 | 16,5±0.7 |
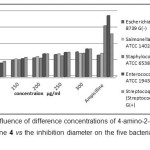 |
Figure 8: The influence of difference concentrations of 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 vs the inhibition diameter on the five bacterial pathogens strains Click here to View Figure |
Antifungal activity
The antifungal activity of the imino fluorinated compound was evaluated by the agar diffusion method at concentrations of 100; 150; 200; 250; 300 µg/ml using Dimethyl Sulfoxide (DMSO) as solvent and ampicillin as standard drug. The results are reported in Table 3 and figure 9 and 10.
From the screening results it is clearly observed that compound 4 showed a slight activity towards Candida albicans at concentration 200; 250 and 300 µg/ml, a mild/moderate activity for the case of concentration 100; 150 µg/ml when compared to Nystatine.
Table 3: Antifungal activity of 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4
| diameters of the inhibition (mm) | ||||||
| Concentration (µg/ml) | Nystatine100µg | 100 | 150 | 200 | 250 | 300 |
| Candida albicansATCC 10231 | 33,5±0.7 | 9±0.7 | 10,75±0.3 | 12,25±1.0 | 14,25±0.3 | 14,75±0.3 |
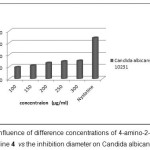 |
Figure 9: The influence of difference concentrations of 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 vs the inhibition diameter on Candida albicans Click here to View Figure |
 |
Figure 10: showing zone of inhibition against all bacterial and fungus pathogens strains Click here to View Figure |
2 (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3
Antibacterial activity
The antibacterial activity of the (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 was evaluated by the agar diffusion method at concentrations of 100; 150; 200; 250 µg/ml using Dimethyl Sulfoxide (DMSO) as solvent and ampicillin as standard drug. Inhibition areas were measured in mm at the end of 24 h of incubation at 35°C. The results are reported in Table 4 and fig. 11.
From the screening results it is clearly observed that compound showed no inhibitory activity for the Staphylococus aureus and Enterococcus feacium at concentration 100 µg/ml and exhibited a slight activity towards Escherichia coli; Streptocoque B and Salmonella typhimurium at concentration 200 and 250 µg/ml, a mild/moderate activity for the case of Staphylococus aureus; Streptocoque B and Enterococcus feacium at concentration 100; 150 and 200 µg/ml when compared to Ampicilline .
Table 4: Antibacterial activity of (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3
| diameters of the inhibition (mm) | |||||
| Concentration (µg/ml) | 100 | 150 | 200 | 250 | Ampicilline |
| Escherichia coliATCC 8739 G(-) | 7±0.0 | 9±0.7 | 10.25±0.3 | 11±0.0 | 12.5±0.7 |
| Salmonella typhimuriumATCC 14028 G(-) | 7±0.3 | 8.75±0.3 | 9.5±0.0 | 10.5±0.0 | 15,5±0.7 |
| Staphylococus aureusATCC 6538 G(+) | – | 8.75±0.3 | 9.5±0.7 | 10±0.7 | 39,5±0.7 |
| Enterococcus feaciumATCC 19434 G(+) | – | 9±0.0 | 10.25±0.3 | 11.5±0.7 | 37±0.7 |
| Streptocoque B G(+)(Streptococcus agalactiae) | 9±0.7 | 12.75±0.3 | 16.25±1.0 | 19.5±0.7 | 35,5±0.7 |
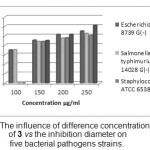 |
Figure 11: The influence of difference concentrations of 3 vs the inhibition diameter on five bacterial pathogens strains. Click here to View Figure |
Antifungal activity
The antifungal activity of the imino fluorinated compound was evaluated by the agar diffusion method at concentrations of 100; 150; 200 and 250 µg/ml using dimethyl sulfoxide (DMSO) as solvent and ampicillin as standard drug. The results are reported in Table 5 and (fig.12).
From the screening results it is clearly observed that compound showed a mild/moderate activity towards Candida albicans at concentration 200 and 250µg/ml when compared to Nystatine.
Table 5: Antifungal activity of (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3
| diameters of the inhibition (mm) | |||||
| Concentration (µg/ml) | 100 | 150 | 200 | 250 | Nystatin |
| Candida albicansATCC 10231 | – | 9±0.0 | 10.75±0.3 | 11.25±0.3 | 35,75 |
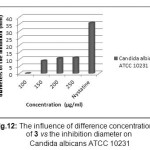 |
Figure 12 a: The influence of difference concentrations of 3 vs the inhibition diameter on Candida albicans ATCC 10231 Click here to View Figure |
 |
Figure 12 b: showing zone of inhibition against all bacterial and fungus pathogens strains Click here to View Figure |
The antibacterial and antifungal activity may be due to the effect of compounds 3 and 4 on the permeability of the cell membrane and the function of the bacterial cell. This can be attributed to the presence carbon-fluorine bond that have inhibitory efficacy on the positive and negative gram bacteria. On the other hand, Schiff bases appear to be important intermediates in a number of enzymatic reaction involving interaction of the amino group of an enzyme, usually that of a lysine residue, with a carbonyl group of the substrate.11 Schiff bases have also a wide range of potential applications in various biological fields,12 also fluorinated Schiff bases derivatives have been widely studied because they have antifungal, antibacterial,1,13,14 DNA cleavage15 and anticancer16 activities which give it attracted remarkable attention. Generally, the two fluorinated compounds (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 are more or less effective towards the tested bacteria.
Conclusion
The reaction of 4-amino tetrafluoropyridine 1 with benzaldehyde 2 in EtOH/ or THF gave 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 and a Schiff base (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 in 33 and 48 % yields respectively. Exploration of the anti-bacterial activity against both gram-positive and gram-negative bacteria showed that these compounds 3 and 4 compounds at a concentration of 250 µg/ml exhibited a slight activity towards Enterococcus feacium; Streptocoque B and Staphylococus aureus exhibited a mild/moderate activity for the case of Escherichia coli and Salmonella typhimurium when compared to Ampicillin. From the results obtained by the antifungal activity, it is found that this fluorinated Schiff base 3 and fluorinated ethoxypyridine 4 are more active against Candida albicans at a concentration of 250 µg/ml when compared to Nystatine.
The results obtained in the present study suggest that the synthesized Schiff base (E)-N-benzylidene-2,3,5,6-tetrafluoropyridin-4-amine 3 and 4-amino-2-ethoxy-3,5,6-trifluoropyridine 4 can be used in treating deseases caused by the tested organisms. Further fluorinated Schiff base investigations may be carried out to synthesize and identify the sites responsible for the antimicrobial activity.
Acknowledgment
The authors wish to express their sincere thanks to Algerian Ministry of Higher Education and Scientific Research (MHESR) for their support and providing the necessary facilities to carry out this research. They are thankful to the staff of INRAP (National Institute of Research and physico-chemical analysis) of Tunisia. Sincere thanks are due to the staff of the analytical and spectroscopic services of the Department of chemistry, University of Manchester for their assistance and providing the necessary facilities.
References
- Alcives Avila-Sorrosa, J. I. H.; Alicia Reyes, A. R.; Reyna, R. M. J.; Roberto, P. M.; David Morales, M. Journal of Molecular Structure 2015, 1085, 249-257.
CrossRef - Bi, H.; Ye, K.; Zhao, Y.; Yang, Y.; Liu, Y.; Wang, Y. Organic Electronics 2010, 11, 1180-1184.
CrossRef - Maienfisch, P.; Hall, R. G. CHIMIA International Journal for Chemistry 2004, 58, 93-99.
- Bagryanskaya, I. Y.; Gatilov, Y. V.; Maksimov, A. M.; Platonov, V. E.; Zibarev, A. V. Journal of Fluorine Chemistry 2005, 126, 1281-1287.
CrossRef - Patani, G. A.; LaVoie, E. J. Chemical reviews 1996, 96, 3147-3176.
CrossRef - Kumar, B. N. P.; Mohana, K. N.; Mallesha, L. Journal of Fluorine Chemistry 2013, 156, 15-20.
CrossRef - Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chemical Society Reviews 2008, 37, 320-330.
CrossRef - Schiff, H. Justus, L. Annalen der Chemie 1864, 131, 118-119.
CrossRef - da Silva, C. M.; da Silva, D. L.; Modolo, L. V.; Alves, R. B.; de Resende, M. A.; Martins, C. V. B.; de Fátima, Â. Journal of Advanced Research 2011, 2, 1-8.
CrossRef - Pang, W.; Zhao, J. W.; Zhao, L.; Zhang, Z. K.; Zhu, S. Z. Journal of Molecular Structure 2015, 1096, 21-28.
CrossRef - Abdul, R.; PhD Thesis, Bahauddin Zakariya University, Multan, 2005.
- Sinha, D.; Tiwari, A. K.; Singh, S.; Shukla, G.; Mishra, P.; Chandra, H.; Mishra, A. K.; European journal of medicinal chemistry 2008, 43, 160-165.
CrossRef - Karthikeyan, M. S.; Prasad, D. J.; Poojary, B.; Bhat, K. S.; Holla, B. S.; Kumari, N. S. Bioorganic & medicinal chemistry 2006, 14, 7482-7489.
CrossRef - Singh, H. L. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2010, 76, 253-258.
CrossRef - Alizadeh, I. Y. R.; Afzal, M.; Srivastav, S.; Srikrishna, S.; Arjmand, F. Journal of Photochemistry and Photobiology B: Biology 2015, 1, 15-21.
- Krzysztof S. A. M.; Osinka. A.; Sztanke, M. Bioorganic & Medicinal Chemistry 2013, 21, 3648-3666.
- Sekhri, L. Asian Journal of chemistry 2005, 17(3), 1747-1766.

This work is licensed under a Creative Commons Attribution 4.0 International License.









