Silver Nanoparticles Green Synthesis using Aqueous Extract of Citrus Reticulate Var Page
Haniyeh Norouzi1, Kambiz Larijani2* and Hossein Behmadi1
1Department of Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran.
2Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: larijani_k@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320450
The silver nanoparticles have great attention for researchers who wish to use them for development of new generation nano devices. In this present study silver nanoparticles were synthesized from aqueous silver nitrate (0.001mM) through a simple and ecofriendly route using leaf extract of Citrus reticulate as reducing agent and stabilizer. The aqueous silver ions when exposed to leaf extract were reduced and resulted in the green synthesis of silver nanoparticles ranges from 44.0 to 54.1 nm. The bio-reduced silver nanoparticles were characterized by UV-Vis spectrophotometer, Energy Dispersive X-ray (EDX), Scanning Electron Microscopic (SEM), and Fourier transform infra-red (FT-IR) spectroscopy.
KEYWORDS:Citrus reticulate; silver nanoparticles; bio-synthesis
Download this article as:| Copy the following to cite this article: Norouzi H, Larijani K, Behmadi H. Silver Nanoparticles Green Synthesis using Aqueous Extract Citrus Reticulate Var Page. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Norouzi H, Larijani K, Behmadi H. Silver Nanoparticles Green Synthesis using Aqueous Extract Citrus Reticulate Var Page. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=18824 |
Introduction
In last decade, study on silver nanoparticles becomes a worldwide interest due to their potential in industrial and biological application such as ceramics, photoelectric, environmental pollution remedy, drug delivery, medicine and chemistry (1). Ag nanoparticles fabrication using chemical (2) and physical procedures could be harmful for environment and living organisms and expensive economically (3) so many researchers tend to fabricate the nanoparticles using biologically methods due to their using eco-friendly, nontoxic and inexpensive materials, especially for when this material used in medical applications (4). As mentioned in literatures, use of biological routes for nanoparticles has advantages of production large amounts of Ag nanoparticles which have a particular size and no contamination (5, 6). The synthesis of nanoparticles can do by two methods, “top down” method and “bottom up” method. Bottom up procedure which using metal salt solution to producing of nanoparticles mainly used in biological route which using microorganism (7) and plant extracts for reduction Ag ions to Ag nanoparticles (8). Hamed et al was reported the biosynthesis of Ag nanoparticles using Haliclona, a marine sponge collected from Persian Gulf, extract at size of 27-46 nm (9). Here is one hypothesis that some phytochemicals in plant extracts such as alkaloids, polyphenols, proteins, carbohydrates and flavonoids can reduce Ag+ ions to Ag0 (10,11,12).
Citrus fruit is a main crop which grown in north and south of Iran and use in Iranian nourishing such as fresh fruit and juices. Citrus peel extract, which represents roughly one half of the fruit mass, is a rich source of bioactive compounds including natural antioxidants such as phenolic acids and flavonoids (13). The most investigations on Citrus reticulate varieties were deal with their essential oil analysis but it had been reported, Hesperidin is the most important flavonoid which found in Citrus reticulate peel extract has a wide range of pharmacological properties (14).
The peel of Citrus reticulate is a waste material after its fruit has been used so the objective of this study is determination the ability of the extract of Citrus reticulate peel water extract which contain bioactive chemical such as flavonoids, for reduction of Ag+ ions to Ag nanoparticles as an eco-friendly and nonexpensive methods in nanotechnology science.
Material and Methods
Plant material
Citrus reticulate peel was collected from Ramsar. Province of Mazadndaran in December, 2014. The plant material was identified by Dr. Golein in Citrus Institute of Iran Rangelands institute of Iran. The water extract solution of Citrus reticulate Peel was prepared by boiling of 5 g of air dried peel tissue of Citrus reticulate in 100 ml deionized water for 10 min. The mixture then filtered using Wattman No.1 filter paper and filtered solution was kept in 4˚C (15).
Synthesis of Silver Nanoparticles
0.001 M aqueous solution of Silver nitrate (AgNO3) was prepared and used for the synthesis of silver nanoparticles. 10 ml of Citrus reticulate extract was added into 90 ml of aqueous solution of 0.001 M Silver nitrate for reduction into Ag+ions to Ag0 particles and kept at room temperature for 5 hours.
Characterization of Ag nanoparticles
UV-Vis Spectra analysis
The reduction of Ag+ ions to Ag0 NPs was monitored by measuring the UV-Vis spectrum of the reaction medium at 5 hours after diluting a small aliquot of the sample into distilled water. UV-Vis spectral analysis was done using Varian Carry 300UV-Vis spectrophotometer from 380 to 550 nm (figure 2).
FT-IR spectral analysis
FT-IR was used for determination of the active functional groups in reducing procedure of Ag+ ions. To remove any free biomass residue or compound that is not the capping ligand of the nanoparticles, the residual solution, after reaction was centrifuged at 13000 rpm for 20 min. and the resulting suspension was re-dispersed in 1 ml sterile deionized water. The centrifuging and re-dispersing process was repeated three times. Thereafter, the purified suspension was freeze dried to obtain dried powder. Finally, the dried nanoparticles were analyzed by a Nicolet Nexus FT-IR spectrophotometer.
Scanning electron microscopy and Energy-dispersive X-ray spectroscopy
The silver nanoparticle solution thus obtained was centrifuged at 13000 rpm for 20 min. such as preparation procedure for FT-IR, the achieved pellet used for SEM, and EDX analyses. Scanning Electron Microscopic (SEM) analysis was done using Leo 440i SEM instrument. Thin films of the sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper and then the film on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 minutes.
The element analysis of the silver nanoparticles was performed using EDX. The freeze-dried silver nanoparticles were mounted on specimen stubs with double-sided taps, and analyzed by EDX 3000 instrument.
Results and Discussion
The change in color of solution from yellow to brownish red showed the reducing of Ag+ to Ag nanoparticles (figure 1). It is well known that silver nanoparticles exhibit brownish red color in aqueous solution due to excitation of surface plasmon vibrations in silver nanoparticles.
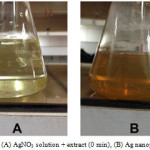 |
Figure 1: (A) AgNO3 solution + extract (0 min), (B) Ag nanoparticles Click here to View Figure |
The Formation of Ag nanoparticles by reduction of the aqueous Ag+ ions can be easily followed by UV–Vis spectroscopy. UV-Vis absorption spectrum of silver nanoparticles shown in figure 2. The Surface Plasmon band in the silver nanoparticles solution remains close to 435 nm throughout the reaction period, suggesting that the nanoparticles were dispersed in the aqueous solution with no evidence for aggregation in UV-Vis absorption spectrum (figure2).
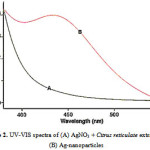 |
Figure 2: UV-VIS spectra of (A) AgNO3 + Citrus reticulate extract and(B) Ag-nanoparticles |
FT-IR analysis was used for the characterization of the extract and the resulting nanoparticles FTIR spectra of water soluble extract before and after reduction of Ag ions are shown in Figure 3.
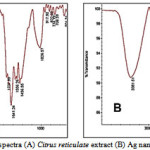 |
Figure 3: FT-IR spectra (A) Citrus reticulate extract (B) Ag nanoparticles + extract |
Absorbance bands in Fig. 3A (before bio-reduction) are observed in the region of 500-2000 cm-1 are 1026, 1058, 1556, 1641, 1737cm-1. These absorbance bands are known to be associated with the stretching vibrations for –C C–C O, –C C– [(in-ring) aromatic], –C–C– [(in-ring) aromatic], C–O (esters, ethers) and C–O (polyols), respectively.
Particular, the 1226 cm-1band arises most probably from the C–O group of polyols such as hydroxyflavones and catechins. The total disappearance of this band after the bioreduction (Fig.3B) may be due to the fact that the polyols are mainly responsible for the reduction of Ag ions, whereby they themselves get oxidized to unsaturated carbonyl groups leading to a broad peak at 1641cm-1(for reduction of Ag).
EDX spectrometers confirmed the presence of elemental silver signal of the silver nanoparticles (Fig. 4) with 27.69% (figure 4). The vertical axis displays the number of x-ray counts whilst the horizontal axis displays energy in KeV. Identification lines for the major emission energies for silver (Ag) are displayed and these correspond with peaks in the spectrum, thus giving confidence that silver has been correctly identified.
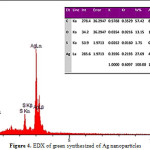 |
Figure 4: EDX of green synthesized of Ag nanoparticles |
Reduction of silver ions present in the aqueous solution of silver complex during the reaction with the ingredients present in the Citrus reticulate extract observed by the UV-Vis spectroscopy revealed the presence of silver nanoparticles may be correlated with the UV-Vis spectra. UV-Vis spectroscopy is well known to investigate shape and size controlled of nanoparticles. The SEM analysis showed the particle size between 33.44 to 44.75 nm as well the cubic structure of the nanoparticles (Figure 5).
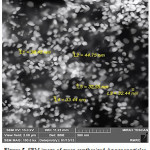 |
Figure 5: SEM image of green synthesized Ag nanoparticles |
FTIR analysis confirmed that the Bio-reduction of Ag+ ions to silver nanoparticles is due to the reduction by capping material of plant extract. The Silver nanoparticles synthesized via green route are highly toxic to multidrug resistant bacteria hence has a great potential in biomedical applications. The present study showed a simple, rapid and economical route to synthesized Silver nanoparticles.
Conclusions
In conclusion, the bio-reduction of aqueous Ag+ ions by the fruit extract of the Citrus reticulate has been demonstrated. The reduction of the metal ions through leaf extracts leading to the formation of silver nanoparticles of fairly well-defined dimensions. In the present study we found that fruits can be also good source for synthesis of silver nanoparticles. This green chemistry approach toward the synthesis of silver nanoparticles has many advantages such as, ease with which the process can be scaled up, economic viability, etc. Applications of such eco-friendly nanoparticles in bactericidal, wound healing and other medical and electronic applications, makes this method potentially exciting for the large-scale synthesis of other inorganic materials (nano materials).
References
- Sougata Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D; Jabgunde, A.; Chopade, B.A. Int. J. Nanomed. 2012, 7, 483–496
- Parsa Mehr, F.; Khanjani, M.; Vatani, P. Orient. J. Chem. 2015, 31, 1831-1833
CrossRef - Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Meera Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P.; Colloids and Surf. B: Biointerfaces, 2012, 96, 69– 74
CrossRef - Bar, H.; Bhui, D.K.; Sahoo G.P.; Sarkar, P.; De, S.P.; Misra, A. Colloids Surf. A 2009, 339, 134 –139
CrossRef - Hutchison, J.E. ACS Nano, 2008, 2, 395–402
CrossRef - Raveendran, P.; Fu, J.; Wallen, S.L. J. Am. Chem. Soc. 2003,125, 13940– 13941
CrossRef - Ghorbani, H.R.; Soltani, S. Orient. J. Chem. 2015, 31, 341-344
CrossRef - Sepeur, S. Nanotechnology: technical basics and applications. Hannover: Vincentz; 2008
- Rezazaeh Hamed, M.; Givianrad, M.H.; Mashinchian Moradi, A. Orient. J. Chem. 2015, 31, 1961-1967
CrossRef - Mohanpuria, P.; Rana, N.K.; Yadav, S.K. J. Nanoparticle. Res. 2008, 10, 507 – 517
CrossRef - Leela, A.; Vivekanandan, M. Tapping the unexploited plant resources for the synthesis of silver nanoparticles. Afr. J. Biotechnol. 2008, 7, 3162 – 3165
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Biotechnol. Adv. 2013, 31, 346– 356
CrossRef - Li, B.B.;, Smith B.;, Hossain M.M. Sep. Purif. Technol. 2006, 48, 182–188.
CrossRef - Ma, Y.; Ye, X.; Hao, Y.; Xu, G.; Xu, G.; Liu, D. Ultrasonics Sonochemistry, 2008, 15, 227–232
CrossRef - Singh, A.; Jain, D.; Upadhyay, M.K.; Khandelwal, N.; Verma, H.N. Digest Journal of Nanomaterials and Biostructures, 2012, 5, 483-489.

This work is licensed under a Creative Commons Attribution 4.0 International License.









