Mg(ClO4)2 as A Recyclable Catalyst for Synthesis of 4H-Chromenes
Masoud Mohammadi Zeydi1*and Somayeh Ahmadi2
1Department of Organic Chemistry, University of Guilan, University of campus 2, Rasht, Iran.
2Department of chemistry, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran.
Corresponding Author E-mail: zedi.65@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320453
Article Received on :
Article Accepted on :
Article Published : 27 Jul 2016
The 4H-choromen derivatives were obtained in excellent yield by three component of an aromatic aldehyde, dimedon and malonitril in the presence catalyst Mg(ClO4)2 as a lewis acid catalyst, in the conditions reflux. The advantage of this method addition to the resuble catalyst, high yield and easy method.
KEYWORDS:4H-choromen; aromatic aldehyde; dimedon; malonitril
Download this article as:| Copy the following to cite this article: Zeydi M. M, Ahmadi S. Mg(Clo4)2 as A Recyclable Catalyst for Synthesis of 4H-Chromenes. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Zeydi M. M, Ahmadi S. Mg(Clo4)2 as A Recyclable Catalyst for Synthesis of 4H-Chromenes. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=19645 |
Introduction
4H-chromenes are important heterocyclic structural with a number of biological and pharmacological properties including anti-cancer [1],anti-tumor [2], anti-proliferative [3], antidiabetic [4], anti-viral [5], and anti-microbial [6], antidepressant[7], activities. They have also been widely employed as pigments, cosmetics [8], and potent biodegradable agrochemicals [9]. Substituted 4H-chromene derivatives are potent apoptosis in ducting agents possessing vascular disrupting activity [10].
In recent years new method have been developed for the synthesis of 4H-choromen derivatives using various reagents such ammonium salts [11-14], amino functionalized silica gel [15], K2CO3 [16], DBU [17], SiO2-Pr-SO3H [18], boric acid [19], Piperidine [20], MgO [21], CuO-CeO2 nanocomposite [22], CeCl3.H2O [23], Methane Sulphonic Acid [24] Potassium Phosphate [25], and different energy sources such as ultrasonic irradiation [26], microwave-irradiation [27,28], ionic liquid [29] and Grinding [30]. Recently, a catalyst free method was reported for the synthesis of chromene derivatives in water [31]. In the study, we report Mg(ClO4)2 as a new lewis acid catalyst for the three component synthesis of 4H-chromene derivatives under conditions reflux.
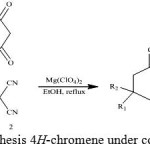 |
Figure 1: Synthesis 4H-chromene under condition reflux |
Results and Discussion
Mg(ClO4)2 were tested as the catalyst in the synthesis of 2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 4a (Figure 1). At the start of this investigation, the one-pot three component reaction of benzaldehyde 1a, malononitrile 2 and dimedon 3 (1 mmol each) in ethanol was employed as a model reaction. The use of different amounts of catalyst (10, 20, 25, and 30W %) at different temperature was investigated. The best result was obtained with 25W% of starch solution at 100 °C (Tables 1, Enter 11).
Table 1: Effect of quantity of Mg(ClO4)2 and temperature on the synthesis of 4a
|
(%)Yield |
(h) Time |
(°C) M.P. |
(W%)Quantity |
Enter |
|
……. |
6 |
……. |
…… |
1 |
|
33 |
6 |
100 |
…… |
2 |
|
35 |
5 |
80 |
10 |
3 |
|
38 |
5 |
90 |
10 |
4 |
|
42 |
5 |
100 |
10 |
5 |
|
39 |
4 |
80 |
20 |
6 |
|
43 |
4 |
90 |
20 |
7 |
|
42 |
4 |
100 |
20 |
8 |
|
63 |
3 |
80 |
25 |
9 |
|
82 |
3 |
90 |
25 |
10 |
|
87 |
3 |
100 |
25 |
11 |
|
78 |
3 |
80 |
30 |
12 |
|
80 |
3 |
90 |
30 |
13 |
|
80 |
3 |
100 |
30 |
14 |
The model reaction was carried out in different solvent such as water, ethanol, methanol, chloroform acetonitrile in the conditions reflux and so solvent free condition at 100 °C using 25 W% of Mg(ClO4)2 as catalyst (table 2). Further, we studied model reaction in water gave the highest yield (87%) of 4a at reflux condition (tables 2, Enter 2).
Table 2: synthesis of 4H-chromenes (4a) in different solvents
|
(%)Yield |
Time (h) |
Solvent |
Enter |
|
45 |
3:30 |
Solvent-free |
1 |
|
63 |
3 |
Water |
2 |
|
87 |
3 |
Aqueous Ethanol |
3 |
|
83 |
3 |
Methanol |
4 |
|
51 |
3:30 |
Chloroform |
5 |
|
54 |
3:30 |
Acetonitrile |
6 |
To study the recyclability of the catalyst, the Mg (ClO4)2 were used for the same reaction repeatedly and the change in their catalytic activity was studied. The relation between the number of the reaction and the catalytic activity in terms of yield of product is presented in table 3. The Mg(ClO4)2 can be recycled and reused as a catalyst for at least four times without losing activity.
Table 3: recyclability of catalyst for the synthesis of 4H-chromene 4a
|
Yield (%) |
Catalyst(g) |
number |
|
87 |
0.057 |
1 |
|
85 |
0.055 |
2 |
|
82 |
0.051 |
3 |
|
72 |
0.045 |
4 |
After optimization of the reaction conditions, the scope and generality of these conditions with other reactants were examined by using a variety of aromatic aldehydes, dimedon and malononitrile. The results are summarized in Table 4. All the reactions proceeded smoothly under the optimized conditions and all the reactions were satisfactorily completed within 2.5–3.5 hour.
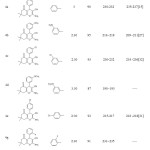 |
Table Click here to View Table |
In summary, a one-pot, three-component condensation reaction of various aldehydes aromatic, dimedon and malononitriles was successfully accomplished to give 4H-chromene derivatives. This methodology has advantages of mild reaction conditions, simple experimentation, and high yields.
Experimental materials and Methods
Compounds were obtained from Merck and used without further purification. The melting points were obtained on an electro-thermal capillary melting point apparatus and are uncorrected. Thin-layer chromatography was performed using HF254 fluorescent silica gel plates (Merck), which were examined under UV 254 and 365 nm light. Infrared spectra (ν/cm-1) were recorded on a Shimadzu IR-470, using KBr disks. 1H spectra were recorded on a DRX-400 MHz NMR Spectrometer at 293 K in CDCl3. Spectra were internally referenced to TMS. Peaks are reported in ppm downfield of TMS.
General procedure for the one-pot preparation of 2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile(4a-l): To a mixture of aldehydes aromatic (1 mmol) in ethanol (5 ml) was added a malononitril(1 mmol), dimedone (1 mmol) and Mg(ClO4)2 (25 mol%). The reaction mixture was stirred for appropriate time as given table 4. After completion of the reaction (monitored by TLC), Mg(ClO4)2 was filtered out using filter paper. Then, the solvent of the filtrate was evaporated and the crude product was purified by recrystallization from hot ethanol. The product were characterized by the comparison of their spectral (1H NMR of IR) and physical data with those reported in the literature.
2-amino-7,7-dimethyl-4-phenyl-5-oxo-5,6,7,8-tetrahydro-4H-chronene-3-carbonitril (4a)
Yellow Crystal. Yield (90%), m.p .230-232 oC. FT-IR (Vmax/cm-1) (KBr disc): 3415, 3339 (NH2 Str.); 3050 (C-Harom Str.); 2990 (C-HaliPh Str.); 2243 (CºN Str.); 1686 (C=O Str.); 1215 (C-O Str.). 1HNMR (400.13 MHz, CDCl3): d 1.05 (3H, s, CH3); 1.13 (3H, s, CH3); 2.24 (2H, AB q, 3J = 16.4, 9.4 Hz, CH2); 2.47 (2H, m, CH2); 4.42 (H, s, CH); 4.57 (2H, s, NH2); 7.19-7.32 (5H, m, CHarom).
2-amino-7,7-dimethyl-4-(3-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chronene-3-carbonitril (4b)
Yellow Crystall. Yield (95%), m.p. 216-218 oC. FT-IR (Vmax/cm-1) (KBr disc): 3410, 3320 (NH2 Str.); 3032 (C-Harom Str.); 2900 (C-HaliPh Str.); 2256 (CºN Str.); 1690 (C=O Str.); 1550, 1347 (N2O Str.). 1HNMR (400.13 MHz, CDCl3): d 1.06 (3H, s, CH3); 1.13 (3H, s, CH3); 2.25 (2H, ABq, 3J = 16.4, 10.4 Hz, CH2); 2.50 (2H, AB q, 3J = 16.8, 4.0 Hz, CH2); 4.54 (H, s, CH); 4.79 (2H, s, NH2); 7.50-8.10 (4H, m, 4CHarom).
2-amino-7,7-dimethyl-4-(3-chlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chronene-3-carbonitril (4c)
White Crystal, Yield (93%), m.p. 220-222 °C. FT-IR (Vmax/cm-1) (KBr disc): 3355, 3298 (NH2 Str.); 3120 (C-Harom Str.); 2956 (C-HaliPh Str.); 2186 (CºN Str.); 1651 (CºN Str.); 1215 (C-O Str.).1H NMR (400.13 MHz, CDCl3): d 1.06 (3H, s, CH3); 1.13 (3H, s, CH3); 2.24 (2H, AB q, 3J = 16.4, 3.2 Hz, CH2); 2.47 (2H, m, CH2); 4.40 (H, s, CH); 4.65 (2H, s, NH2); 7.17-7.28 (4H, m, 4CHarom).
2-amino-7,7-dimethyl-4-(3-methoxyophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chronene-3-carbonitril (4d)
White Crystal, Yield (87%), m.p. 190-193 °C. FT-IR (Vmax/cm-1) (KBr disc): 3379, 3301 (NH2 Str.); 3085 (C-Harom Str.); 2965 (C-HaliPh Str.); 2288 (CºN Str.); 1670 (C=O) Str.); 1219 (C-O Str.).1H NMR (400.13 MHz, CDCl3): d 1.07 (3H, s, CH3); 1.13 (3H, s, CH3); 2.25 (2H, ABq, 3J =16.4, 2.4 Hz, CH2); 2.46 (2H, m, CH2); 3.80 (3H, s, CH3); 4.39 (H, s, CH); 4.57 (2H, s, NH2); 6.74-7.28 (4H, m, 4CHarom).
2-amino-7,7-dimethyl-4-(4-chlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chronene-3-carbonitril (4e)
White Crystal, Yield (93%), m.p .215-217 °C. FT-IR (Vmax/cm-1) (KBr disc): 3371, 3303 (NH2 Str.); 3110 (C-Harom Str.); 2980 (C-HaliPh Str.); 2198 (CºN Str.); 1668 (C=O) Str.); 1217 (C-O Str.). 1H NMR (400.13 MHz, CDCl3): d 1.04 (3H, s, CH3); 1.13 (3H, s, CH3); 2.23 (2H, ABq, 3J =16.4, 7.2 Hz, CH2); 2.47 (2H, s, CH2); 4.40 (H, m, CH); 4.58 (2H, s, NH2); 7.18-7.29 (4H, m, CHarom).
2-amino-7,7-dimethyl-4-(2-chlorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chronene-3-carbonitril (4f)
White Crystal, Yield (90%), m.p. 205-207 °C. FT-IR (Vmax/cm-1) (KBr disc): 3320, 3272 (NH2 Str.); 3117 (C-Harom Str.); 2988 (C-HaliPh Str.); 2201 (CºN Str.); 1688 (C=O) Str.); 1215 (C-O Str.). 1H NMR (400.13 MHz, CDCl3): d 1.08 (3H, s, CH3); 1.12 (3H, s, CH3); 2.22 (2H, ABq, 3J =16.4, 8.4 Hz, CH2); 2.47 (2H, s, CH2); 2.47 (2H, s, CH2); 4.67 (2H, s, NH2); 4.86 (H, s, CH); 7.13-7.34 (4H, m, 4CHarom).
2-amino-7,7-dimethyl-4-(2-flourophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chronene-3-carbonitril (4g)
White Crystal, Yield (91%), m.p. 233-235 °C. FT-IR (Vmax/cm-1) (KBr disc): 3400, 3350 (NH2 Str.); 3045 (C-Harom Str.); 2950 (C-HaliPh Str.); 2210 (CºN Str.); 1620 (C=O) Str.); 1225 (C-O Str.).1H NMR (400.13 MHz, CDCl3): d 1.06 (3H, s, CH3); 1.13 (3H, s, CH3); 2.24 (2H, ABq, 3J =16.4, 5.6 Hz, CH2); 2.47 (2H, m, CH2); 4.42 (H, s, CH); 4.55 (2H, s, NH2); 7.21-7.32 (4H, m, 4CHarom).
2-amino-7,7-dimethyl-4-(4-methylphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chronene-3-carbonitril (4h)
White Crystal, Yield (89%), m.p. 225-227 °C. FT-IR (Vmax/cm-1) (KBr disc): 3435, 3330 (NH2 Str.); 3095 (C-HaromStr.); 2964 (C-HaliPh Str.); 2201 (CºN Str.); 1630 (C=O) Str.); 1205 (C-O Str.). 1H NMR (400.13 MHz, CDCl3): d 1.06 (3H, s, CH3); 1.12 (3H, s, CH3); 2.23 (2H, ABq, 3J =16.4, 5.6 Hz, CH2); 2.30 (3H, s, CH3); 2.46 (2H, s, CH2); 4.38 (H, s, CH); 4.55 (2H, s, NH2); 7.09-7.28 (4H, m, CHarom).
Conclusions
We report a novel one‐pot three‐component synthesis of functionalized 4H-chromene derivatives in the presence of Mg(ClO4)2 as catalyst. This reaction series was found to be highly effective under reflux conditions. Mg(ClO4)2 is an effective catalyst and provides a new and useful method for the synthesis of pyrannulated heterocyclic systems by condensation of arylaldehydes, dimedonand malononitrile. The catalyst is environmentally friendly, inexpensive, clean, safe, nontoxic, and easily obtained. Moreover, the procedure offers several advantages including high yields, clean reaction conditions, and no pollution threat to the environment, which together make a useful and attractive process for synthesis of these compounds.
Acknowledgments
The partial support of this research by the Research Committee of the University of tonekabon Islamic Azad University is gratefully acknowledged.
References
- Zhang, G.; Zhang, Y.; Yan, J.; Chen, R.; Wang, S.; Ma, Y.; Wang, R. J. Org. Chem. 2012, 77, 878-888.
CrossRef - Ahmed, M. E.; Ahmed, M. F.; Alanood, M. A. Med. Chem. Res. 2014, 23, 3187-3199.
CrossRef - Kumar, A.; Sharma, S.; Maurya, R. A.; Sarkar, J. J. Comb. Chem. 2010, 12, 20–24.
CrossRef - Rapposelli, S.; da Settiomo, F.; Digiacomo, M.; la Motta, C.; Lapucci, A.; Sartini, S.; Vanni, M. Arch. Pharm. (weinheim) 2011, 344, 372-385.
CrossRef - Conti, C.; Proietti Monaco, L.; Desideri, N. Bioorg. Med. Chem. 2014, 22, 1201-1207.
CrossRef - Mungra, D. C.; Patel, M. P.; Rajani, D. P.; Patel, R. G. Eur. J. Med. Chem. 2011, 46, 4192-4200.
CrossRef - He, Y.; Chen, Y. Y.; Shi, J. B.; Tang, W. J.; Pan, Z. X.; Dong, Z. Q.; Song, B. A.; Li, J.; Liu, X. H. Bioorg. Med. Chem. 2014, 22, 3732-3738.
CrossRef - Ellis, G. P. In The Chemistry of Heterocyclic Compounds. Chromenes, Harmones, and Chromones; Weissberger, A., Taylor, E. C., Eds.; John Wiley: New York, 1977; 11-139. Chapter II.
CrossRef - Hafez, E. A.; Elnagdi, M. H.; Elagamey, A. A.; El-Taweel, F. A. M. Heterocycles 1987, 26, 903.
CrossRef - Kemnitzer, W.; Kasibhatla, S.; Jiang, S.; Zhang, H,; Wang, Y.; Zhao, J.; Jia, J.; Herich, J.; Labregue, D.; Storer, R.; Meerovitch, K.; Bouffard, D.; Reg, R.; Denis, R.; Blais, C.; Lamothe, S.; Attardo, G.; Gourdeau, H.; Tseng, B.; Drewe, J.; Cai, S. X. j. med. Chem. 2004, 47, 6299-6310.
- Ranu, B. C.; Banerjee, S.; Roy, S. Indian J. Chem. 2008, 47, 1108-1112.
- Shi, D.; Mou, J.; Zhuang, Q.; Wang, X. J. Chem. Res. 2004, 12, 821-823.
CrossRef - Gao, S. J.; Tsai, C. H.; Tseng, C.; Yao, C. F. Tetrahedron 2008, 64, 9143–9149.
CrossRef - Chen, L.; Li, Y. Q.; Huang, X. J.; Zheng, W. J. Heteroat. Chem. 2009, 20, 91-94.
CrossRef - Vijaykumar, M. J.; Rupali, L. M.; Prashant, B. Throat.; Sunil, U. Tekale.; Bhagavan, R. Patil.; Mangal, P. Kale.; Rajendra, P. Pawar. Chin. Chem. Lett. 2014, 25, 455-458.
- He, Y.; Hu, R.; Tong, R.; Li, F.; Shi, J.; Zhang, M. Molecules 2014, 19, 19253-19268.
CrossRef - Zonouzi, A.; Mirzazadeh, R.; Safavi, M.; K Ardestani, S.; Emami, S.; Foroumadi, A. Iran. J. Pharma. Res. 2013, 12, 679-685.
- Mohammadi Ziarani, G.; Badiei, A.; Dashtianeh, Z.; Hajiabbasi, P. Rev. Roum. Chim. 2013, 58, 765-772.
- Adibi, H.; Khodarahmi, R.; Mansouri, K.; Khaleghi, M.; Maghsoudi, S. Pharm. Sci. 2013, 19, 23-30.
- Mahdavi, M.; Asadipour, A.; Rajabalian, S.; Vosooghi, M.; Firoozpour, L.; Nakhjiri, M.; Shafiee A.; Foroumadi, A. E-Journal Chem. 2011, 8, 598-602.
CrossRef - Safari, J.; Zarnegar, Z.; Heydarian, M. J. Taibah Univ. Med. Sci. 2013, 7, 17-25.
CrossRef - Albadi, J.; Razeghi, A.; Mansournezhad, A.; Azarian, Z. J. Nanostructure Chem.2013, 3, 85.
CrossRef - Sabitha, G.; Arundhathi, K.; Sudhakar, K.; Sastry, B. S.; Yadav J. S. Synth. Commun. 2009, 39, 433-442.
CrossRef - Kulkarni, P. Orient. J. Chem. 2015, 31, 447-451.
CrossRef - Pore, D. M.; Undale, K. A.; Dongare, B. B.; Desai, U. V. Cata. Lett. 2009, 132, 104-108.
CrossRef - Safari, J.; Heydarian, M.; Zarnegar, Z. Arab. J. Chem. 2013 in press. http://dx.doi.org/10.1016/j.arabjc.2013.11.038.
CrossRef - Sangani, C. B.; Shah, N. M.; Patel, M. P.; Patel, R. G. J. Serb. Chem. Soc. 2012, 77, 1165-1174.
CrossRef - Nirav, K. S.; Nimesh, M. S.; Manish, P. P.; Ranjan. G. P. J. Chem. Sci. 2013, 125, 525-530.
- Poor Heravi, M. R.; Amirloo, M. R. Iran. Chem. Commun. 2015, 3, 73-84.
- Akandi, A. S.; Balali, E., Mosavat, T.; Ghanbari, M. M.; Eazabadi, A. Orient. J. Chem. 2014, 30, 587-591.
CrossRef - Dekamin, M. G.; Eslami, M.; Maleki, A. Tetrahedron 2013, 69, 1074-1085.
CrossRef - Maghsoodlou, M. T.; Safarzaei, M.; Mousavi, M. R.; Hazeri, N.; Aboonajmi J.; Shirzaei. M. Iran. J. Org. Chem. 2014, 6, 1197-1202.

This work is licensed under a Creative Commons Attribution 4.0 International License.









