Melanogenesis Inhibitory Activity of Rhododendron Weyrichii in Mouse B16 Melanoma Cells.
Min-Jin Kim1, Sang Suk Kim2, Suk Hyun Yun2, Seung-Young Kim3, Kwang Hee Hyun4, Juyeong Lee1, Nam Ho Lee1, and Chang-Gu Hyun1*
1Cosmetic Sciences Center, Department of Chemistry and Cosmetics, Jeju National University, Jeju63243, Korea.
2Citrus Research Institute, National Institute of Horticulture and Herbal Science, RDA, Seogwipo 63607, Korea.
3Department of BT-Convergent Pharmaceutical Engineering, Sun Moon University, Chungnam 31460, Korea.
4Helios Co., Ltd., Sanchundan Dong-gil 16, Jeju 63243, Korea.
*Corresponding Author E-mail: cghyun@jejunu.ac.kr
DOI : http://dx.doi.org/10.13005/ojc/320416
In this study, to evaluate the usefulness of Rhododendron weyrichii Maxim.as a whitening agent, the whitening effects of its extracts were investigated in alpha-melanocyte-stimulating hormone (α-MSH)-induced B16F10 melanoma cells. No toxicity was noted in either B16F10 melanoma cells or HaCaT keratinocyte cells that were exposed to the hot water or 70% ethanol extracts of R. weyrichii Maxim. (RW-H and RW-E, respectively).Moreover, both the RW-H and RW-E extracts dose-dependently inhibited α-MSH-induced melanin production in B16F10 melanoma cells, with inhibitory effects of 52.5% and 51.6%, respectively, at a concentration of 200μg/mL. The RW-H and RW-E extracts also inhibitedintracellular tyrosinase activity in a dose-dependent fashion. Western blot analyses showed that the RW-H and RW-E extracts decreased tyrosinase, tyrosinase-relatedprotein-1, and tyrosinase-relatedprotein-2 expression.Additionally,we found that ρ-coumaric acid-containing RW-H and RW-E extracts could be used as hypopigmentation agentssince they suppress melanogenesis. Collectively, our results suggest that RW-H and RW-E extracts have the potential to serve as functional cosmetic agents, including whitening agents.
KEYWORDS:B16F10 melanoma cell; melanin; melanogenesis; Rhododendron weyrichiiMaxim; tyrosinase; whitening
Download this article as:| Copy the following to cite this article: Min-Jin K, Kim S. S, Yun S. H, Seung-Young K, Hyun K. H, Lee J, Lee N. H, Chang-Gu H. Melanogenesis Inhibitory Activity of Rhododendron Weyrichii in Mouse B16 Melanoma Cells. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Min-Jin K, Kim S. S, Yun S. H, Seung-Young K, Hyun K. H, Lee J, Lee N. H, Chang-Gu H. Melanogenesis Inhibitory Activity of Rhododendron Weyrichii in Mouse B16 Melanoma Cells. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20708 |
Introduction
Melanin plays an important role in protecting human skin from the harmful effects of ultravioletradiation from the sun. In addition, melanin determines our phenotypic appearance. Melanin biosynthesis proceeds through acomplex series of enzymatic and chemical reactions in melanocytes1-4and is regulated bymelanogenic factors such as tyrosinase, tyrosinase-relatedprotein-1 (TRP-1), and tyrosinase-related protein-2 (TRP-2)5.Tyrosinase is particularly important as itcan catalyze three different reactions, namely the hydroxylation of tyrosineto 3,4-dihydroxyphenylalanine (DOPA), the oxidationof DOPA to DOPA quinone, and the oxidation of 5,6-dihydroxyindole(DHI) to indole-quinone6.In the absence of thiols,DOPA quinone changes to DOPA chrome and then toDHI or indole 5,6-quinone 2-carboxylic acid (DHICA).Two additional steps in this melanogenic pathway include theconversion of DOPA chrome to DHICA, which is catalyzedby TRP-2 (DOPA chrome tautomerase), and the oxidation of DHICA, which is catalyzed by TRP-1 (DHICA oxidase). Moreover, microphthalmia-associated transcription factor (MITF) strongly stimulates tyrosinase, TRP-1, and TRP-2, indicating that itis an important regulator of melanogenesis7-11.
Although melanin has mainly photoprotective functions in human skin, the accumulation of anabnormal amount of melanin in different parts of the skin can create differentially pigmented patches, whichmay be an esthetic problem for some individuals. This phenomenon has encouraged researchers to discover new potenttyrosinase inhibitors that can inhibit tyrosinase activity, and in turn reduce melanogenesis, in order to serve as skin-whitening cosmetics.
Rhododendron weyrichiiMaxim.is a deciduous shrub of the Ericaceae family and its only known habitat is Jeju Island in South Korea.Rhododendron plants have been mostly used as folk medicines to treathigh blood pressure or increase urine production.For example,the leaves of Rhododendron schlippenbachii and Rhododendronyedoensevar.poukhanense have been used to treat the early symptoms of hypertension, while the leaves ofRhododendron brachycarpum have been used as a diuretic12. However, the pharmacological effects of R. weyrichiiMaxim.have not been examined scientifically. Therefore, in the presentstudy, we verified the pharmacological whitening effects of R. weyrichiiMaxim.inα-MSH-induced B16F10 murine melanoma cells.
Materials and Methods
Plant Material
R. weyrichii Maxim.flowers were collected from JejuNational University in May, 2015. The materials for extraction were freeze-dried, and then ground into a fine powder by using a blender. The dried powder (1 kg) was extracted with hot water (water, 1L) at 70°C for 4 h (hot-water extract [RW-H]), and then evaporated under a vacuum. All other extracts (EtOH extracts [RW-E]; 1 L, 1 kg) except the RW-H extracts were extracted at room temperature for 2 days. The yieldof the RW-H, 20%, 50%, 70%, and 100% RW-E extracts were 14.4%, 39.6%, 30.1%, 22%, and 17.6%, respectively.
Cell Culture
The B16F10 murine melanoma cell line was purchased from the Korean Cell Line Bank (Seoul, Korea). These cells were maintained at sub-confluence in a 95% air/5% CO2 humidified atmosphere at 37°C. The medium used for the routine subculture was Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 μg/mL).
MTT Assay
Cell viability was determined using 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assays13,14. B16F10 cells were cultured in 24-well plates for 18 h, followed by treatment with various concentrations (200, 400, and 800μg/mL) of the R. weyrichiiMaxim. extracts for 48 h. Briefly, MTT was added to the cells and the formazan crystals were dissolved in dimethyl sulfoxide. The absorbance was measured at 540 nm. The percentage of cells showing cytotoxicity was determined relative to that in the control group.
Measurement of Melanin Content
The amount of melanin in B16F10 cells was measured according to a previously published method15 with slight modifications. The cells were treated with theR. weyrichiiMaxim. extracts and α-MSH for 48 h at 37°C. After removing the media,the cells were washed with cold phosphate-buffered saline and the cell pellets were dissolved in 1 MNaOH for 1 h at 80°C. Spectrophotometric analysis of the melanin content was performed at an absorbance of 405 nm. Each experiment was performed in triplicate.
Intercellular Tyrosinase Activity
Intercellular tyrosinase activity was determined using methodsdescribedpreviously with slight modifications15. Here, arbutin was used as a positive control. Briefly, B16F10 cells (1.0 × 105) were seeded in a 60-mm dish, and then the plates were incubated at 37°C in a humidified atmosphere of 5% CO2 for 3 days. Next,seeded B16F10 cells were lysed with phosphate buffer containing 1% Triton X-100. The lysates were clarified by centrifugation for 15 min at 13,000 rpm. Afterquantifying the protein levels and adjusting the concentrations usinglysis buffer, we added each lysate, which contained identical amounts of protein, andplaced the indicated concentrations of the test samples into a 96-well plate; then,15 mM L-DOPA was added per well.After incubation at 37°C, we measuredthe absorbance at a wavelength of 475 nm using an enzyme-linked immunosorbent assay reader.
Western Blot Analysis
After the α-MSH-induced B16F10 cells were incubated for 48 h, the cells were washed twice with cold phosphate-buffered saline. The cells were allowed to lyse in lysis buffer (radioimmunoprecipitation assay buffer, 1% Nonidet P-40,1% protease inhibitor cocktail) for 1 h, and were then collected in microtubes and centrifuged at 15,000 rpm for 15 min at 4°C. The supernatants were prepared in new microtubes. The protein content of the cell lysates was determined with Bradford reagent (Bio-Rad) using bovine serum albumin as the standard. After heating at 70°C for 10 min, equal amounts of the cell lysates were separated with 4~12% Bis-Tris mini gel electrophoresis (Invitrogen Inc.) and transferred to a nitrocellulose membrane(Invitrogen Inc.). The membrane was then washed with Tris-buffered saline (TBS; 20 mMTris base, 137 mMNaCl, pH 7.6) containing 0.1% Tween 20 (TTBS) and blocked in TTBS containing 5% skim milk solution for 24 h. The membrane was incubated overnight at 4°C with the primary antibodies diluted in TTBS (1:1000). Primary antibodies (TRP-1, TRP-2, tyrosinase) were purchased from Santa Cruz Inc. Membranes were washed four times with TTBS. Then, each membrane was incubated for 1 h with secondary peroxidase-conjugated goat immunoglobulin G (1:5000) and washed four times with TTBS. The target proteins were detected using an enhanced chemiluminescence solution. The immunoreactive bands were detected and exposed to X-ray film. Protein levels were quantified by scanning the immunoblots.
High-Performance Liquid Chromatography Analysis
High-performance liquid chromatography (HPLC) was performed with a Waters 2695 chromatograph system (Waters, Milford, MA, USA), which included a Waters 2489 UV visible detector. Utilizing a YMC ProC18 column(250 × 4.6 mm)was used to separate ρ-coumaric acid from RW-H and RW-E extracts.The mobile phase of quantitative analysis was a mixture of water–methanol–glacial acetic acid (80:18:2).The injection volume of the sample was10μL.The flowrate was kept at 0.6 mL/min and detection of the ρ-coumaric acid was performedat 320 nm. All solutions,including samples (RW-H and RW-E extracts) andstandard (ρ-coumaric acid),were filtered througha0.45-μmmembrane before being directly injected in to theHPLCsystem.
Statistical Analysis
All data were obtained in triplicate and are presented as the mean ± the standard error of the mean. Significant differences between treatments were determined with Student’s t-tests in one-way analyses of variance.
Results
Effects of R. Weyrichii Maxim.Extracts on B16F10 Melanoma Cell Viability and Hacat Keratinocyte Cellcytotoxicity
To evaluate the effects of theR. weyrichiiMaxim.extracts on cell viability, we treated B16F10 melanoma cells with the RW-H extract and various concentrations of the RW-E extract,and then measured the cell viability using an MTT assay. As shown in Fig. 1A,none of theextracts at concentrationsof up to 200 μg/mLhad significant effects on the cell viability of B16F10 melanoma cells when compared tountreated control cells after 48 h.
In addition, to determine the effects of these extracts on skin-related cells, the cytotoxicity of the extracts toward human HaCaT keratinocyte cells was examined under the same conditionsas those for the B16F10 melanoma cells. As shown in Fig. 1B, none of the extract concentrations used in this study had cytotoxic effects on HaCaT keratinocyte cells. These results indicate that the RW-H and RW-E extracts are safe for human skin cells at these concentrations. Based on the results of the cell viability experiments, the concentration of 200 μg/mL was used in the remaining experiments.
 |
Figure 1: Effects of the Rhododendron weyrichii Maxim.extracts on the cell viability of HaCaT and B16F10 cells. Click here to View Figure |
An MTT assay was performed after incubating the HaCaT and B16F10 cells with the R. weyrichii Maxim. extracts for 24 h at 37°C in a 5% CO2 atmosphere. Cell viability in (A) HaCaT cells and (B) B16F10 cells. Absorbance was measured at 540 nm.
Effects of R. Weyrichii Maxim.Extracts on Melanin Content and Intracellular Tyrosinase Activity in B16F10 Melanoma Cells
Next, we investigated the effects of the RW-H and RW-E extracts on the α-MSH-induced melanin content in B16F10 melanoma cells. Cells treated with α-MSH alone had markedly increased melanin content compared to untreated cells. All RW-H and RW-E extracts(200 μg/mL) significantly inhibited the melanin content better than didarbutin(Fig. 2A).
The observed suppression of the melanin indicates that they are associated with the activity of the enzyme involved in melanin synthesis. Tyrosinase is the rate-limiting step (reaction rate-determining step) in the melanin synthesis process and is responsible for the first stage of melanin biosynthesis16. This suggests that inhibiting the activity of tyrosinase may also inhibit melanin synthesis.Therefore, we examined whether the RW-H and RW-E extracts would affect the activity of tyrosinase by treating B16F10 cells with the extracts and measuring the tyrosinase activity.The extracts inhibited tyrosinase to a degree that was similar to the amount of melanin synthesis(Fig. 2B).
Thus, the RW-H and RW-E extracts inhibited the melanin content and intercellular tyrosinase activity. Since the experiment was performed by selecting the RW-H and 70% RW-Eextracts, we next examined their potential as cosmetic raw materials.
 |
Figure 2: Inhibitory effects of Rhododendron weyrichii Maxim.extracts on melanogenesis and intracellular tyrosinase activity in B16F10 cells. Click here to View Figure |
B16F10 cells (2.0 × 104) were pre-incubated for 18 h and the melanin content was examined in B16F10 cells treated with α-MSH (200 nM), arbutin (100 μM), and the R.weyrichii Maxim. extracts for 72 h at 37°C in a 5% CO2 atmosphere. The melanin content (A) and cellular tyrosinase activity (B) were measured as described in the ‘‘Materials and methods’’ section.
Effects of RW-H And RW-Eon the Melanin Content and Intracellular Tyrosinase Activity in B16F10 Melanoma Cells
As mentioned above, neither the RW-H nor RW-E extracts were toxic to B16F10 cells at concentrations of 50 μg/mL to 200 μg/mL (Fig. 1). Next, we explored the effects of the RW-H and RW-E extracts on α-MSH-induced melanin synthesis and tyrosinase activity. As shown in Fig. 3, cells treated with α-MSH alone had markedly increased melanin content compared to normal cells. Arbutin, a potent inhibitor in standard skin-whitening agents, was used as a positive control. RW-H and RW-E extracts (200 μg/mL) clearly inhibited melanin synthesis more than did arbutin. In fact, the RW-H and RW-E extracts dose-dependently inhibited α-MSH-induced melanin production in B16F10 melanoma cells, with inhibitory effects of 52.5% and 51.6%, respectively, at a concentration of 200μg/mL.
Moreover, because melanin synthesis is ultimately regulated by tyrosinase, the direct inhibitory effects of the RW-H and RW-E extracts on tyrosinasewere examined in B16F10 melanoma cellsusing intercellular tyrosinase assays. As shown in Fig. 4, the RW-H and RW-E extracts decreased the activity of intercellular tyrosinasein a concentration-dependent manner.
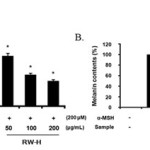 |
Figure 3: Inhibitory effects of Rhododendron weyrichii Maxim.extracts on melanogenesis in B16F10 cells. Click here to View Figure |
B16F10 cells (2.0 × 104) were pre-incubated for 18 h and the melanin content was examined in B16F10 cells treated with α-MSH (200 nM), arbutin (100 μM), and the R. weyrichii Maxim. hot water (RW-H) and EtOH (RW-E) extracts for 72 h at 37°C in a 5% CO2 atmosphere. The melanin content was measured as described in the ‘‘Materials and methods’’ section.
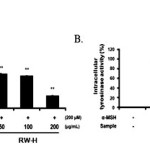 |
Figure 4: Inhibitory effects of Rhododendron weyrichii Maxim.extracts on intracellular tyrosinase activity in B16F10 cells. Click here to View Figure |
B16F10 cells (2.0 × 104) were pre-incubated for 18 h and the melanin content was examined in B16F10 cells treated with α-MSH (200 nM) and the R.weyrichii Maxim. hot water (RW-H) and EtOH (RW-E) extracts for 72 h at 37°C in a 5% CO2 atmosphere. The intracellular tyrosinase activity was measured as described in the ‘‘Materials and methods’’ section.
Effects of RW-H and RW-E on Melanogenesis-Related Proteins in B16F10 Melanoma Cells
The above results suggest that the RW-H and RW-E extracts have inhibitory effects on melanin generation, as the extracts inhibited the tyrosinase activity in B16F10 cells.Thus, we next investigated whether the anti-melanogenic effects of the RW-H and RW-E extracts exert their whitening activity by inhibiting protein expression or function at any other steps in the generation process.
After exposing B16F10 cells to the RW-H and RW-E extracts, we confirmed the expression of tyrosinase, TRP-1, and TRP-2, known as the key enzymes/proteins in melanin production, through western blots.As shown in Fig. 5, when the cells were stimulated withα-MSH, a significant increase in tyrosinase was observed, and TRP-1 and TRP-2 expression also increased. Interestingly, as the concentration of the RW-H extract increased, the amount of tyrosinase and TRP-1 expressiondecreased (Fig. 5A).However, TRP-2 expression did not show a significant change.Incontrast, the RW-E extract did not affect the expression of TRP-1 protein, but did inhibit theα-MSH-induced tyrosinaseand TRP-2 expression in a dose-dependent manner (Fig. 5B). No changes in the protein levelsof β-actin, a housekeeping protein that was used as an internal control, were observed.
Thus, we investigated the inhibition of tyrosinase, TRP-1, and TRP-2 expression by western blotting to describe the influence of the RW-H and RW-Eextracts on α-MSH-induced MITF expression.
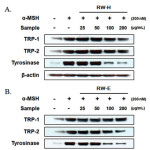 |
Figure 5: Inhibitory effects of Rhododendron weyrichii Maxim.extracts on the levels of tyrosinase, TRP-1, and TRP-2 in α-MSH-induced B16F10 cells. Click here to View Figure |
B16F10 cells (1.0 × 105 cells/mL) were pre-incubated for 18 h, and the cells were stimulated with α-MSH (200 nM) and the R.weyrichii Maxim. hot water (RW-H) and EtOH (RW-E) extracts for 24 h. The expression level was determined using immunoblotting methods.
HPLC Fingerprinting Analysis of The RW-H and RW-E Extracts
Chromatographic methods (for example, HPLC fingerprinting) can be used to identify the active ingredients of traditional oriental medicines. In the field of natural chemistry, interest in HPLC fingerprinting analysis has increased, both in Asia and globally. Therefore, a simple HPLC fingerprinting method was developed. Because p-coumaric acid is reportedly an effective anti-melanogenic agent, it was used as a standard substance. Using HPLC fingerprinting, p-coumaric acid was resolved from the RW-H and RW-E extracts with clear peak shapes. The p-coumaric acid content in the RW-H and RW-E extracts (100 g) was 1.94 g and 14.8 g, respectively (Fig. 6).
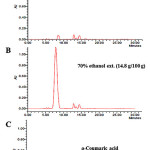 |
Figure 6: HPLC fingerprinting analysis of the R. weyrichii water (A) and 70% ethanol (B) extracts. Click here to View Figure |
The lower side (C) represents standard ρ-coumaric acid. The wavelength of ρ-coumaric acidabsorbance is 320 nm.
Discussion
R. weyrichiiMaxim.is a deciduous shrub that only grows on Jeju Island in South Korea. Rhododendron plants tend to be used as folk medicines to increase urine production or treat high blood pressure.However, the pharmacological effects of R. weyrichiiMaxim.have not been proven scientifically12. Therefore, in the presentstudy, we verified the pharmacological whitening effects of R. weyrichiiMaxim.inα-MSH-induced B16F10 melanoma cells.
In this study, we first determined the potential cytotoxicity of all R. weyrichiiMaxim.extractson B16F10 melanoma cells and HaCaT keratinocyte cells by using MTT assays. The results showed that cells retained almost the same viability when exposed to each extract at concentrations of 200 μg/mL.
Next, the inhibitory action of each extract on melanogenesis was evaluated. Melanin synthesis is mainly activated by ultraviolet light, which generates DNA photoproducts and causes various autocrine and paracrine factors to be secreted. One of the most well known factors is α-MSH17. By inducing melanin synthesis withα-MSH, we investigated how the R. weyrichiiMaxim. extractsaffect melanin formation. We found that cells treated with α-MSH alone had markedly increased melanin content compared to normal cells. All R. weyrichiiMaxim.extracts of RW at a concentration of 200 μg/mL significantly inhibited the melanin content better than did arbutin. Moreover, the degree to which the melanin content was suppressed indicates that the extracts are associated with the enzymatic activity of melanin synthesis.
Tyrosinase catalysis is involved in the first two steps of mammalian melanogenesis, which is the process that producesthe dark macromolecular pigment melanin. Over-activity of this enzyme leads to the overproduction of melanin, which inturn produces hyperpigmentation of the skin. Inhibition of tyrosinase can also lead to reduced melanin content. Tyrosinase inhibitors have become increasingly important in medications and cosmetics, as they can prevent hyperpigmentation by inhibiting enzymatic oxidation18,19.Therefore, we determined how the Rhododendron weyrichiiMaxim.extractsaffected the activity of tyrosinase in B16F10 cells.Our results showed that the extracts had inhibitory effects on tyrosinase that were similar to the level of melanin synthesis. Indeed, allextracts examined inhibited the melanin content and intercellular tyrosinase activity.
Next, we selected the RW-H and 70% RW-E extracts and examined their potential to be developed as cosmetic raw materials. We found that the RW-H and RW-E extracts were nottoxic to B16F10 cells at concentrations of 50 to 200 μg/mL. We also explored the effects of the RW-H and RW-E extracts on α-MSH-induced melanin synthesis and tyrosinase activity. The RW-H and RW-E extracts (200 μg/mL) clearly inhibited melanin synthesis better than did arbutin. RW-H and RW-E concentration-dependently inhibited α-MSH-induced melanin production in B16F10 melanoma cells (inhibitory effects of 52.5% and 51.6%, respectively) and decreased the intercellular tyrosinase activity in a concentration-dependent manner.
Melanogenesis is known to be controlled by an enzymatic cascade, which is regulated at the level of tyrosinase, TRP-1, and TRP-220. Thus, to better understand the molecular mechanisms underlying the anti-melanogenic effects of the RW-H and RW-E extracts, we evaluated the expression levels of tyrosinase, TRP-1, and TRP-2. We noted that as the RW-H extract concentration increased the amount of tyrosinase and TRP-1 expression decreased, while the expression level of TRP-2 remained unchanged. On the other hand, the RW-E extract had no effect on TRP-1 expression but did inhibit theexpression of α-MSH-induced tyrosinase and TRP-2 in a concentration-dependent manner. The protein level of β-actin, which was used as an internal control, did not change.
MITF is a transcription factor that effectively transactivatestyrosinase and its related genes by binding to their common promoters21.Hence, we investigated the inhibition of tyrosinase, TRP-1, and TRP-2 expression by western blotting to describe the influence of the RW-H and RW-Eextracts on α-MSH-induced MITF expression.
In conclusion, our results suggest thatthe RW-H and RW-E extracts inhibited melanin production in α-MSH-induced B16F10 melanoma cells by suppressing tyrosinase, TRP-1, and TRP-2 expression. Such inhibitory effects indicate that the RW-H and RW-E extracts can influence MITF protein expression. Furthermore,RW-H and RW-E extracts containing p-coumaric acid could be used as hypopigmentation agentsgiven their ability to suppress melanogenesis. Collectively, our results suggest that RW-H and RW-E extracts could be developed into functional cosmetic agents, such as whitening agents.
Acknowledgements
This work was supported by the Academic and Research Institutions R&D Program (C0268105) funded by the Small and Medium Business Administration (SMBA, Korea).
References
- Hearing, V.J.; Körner, A.M.; Pawelek, J.M.;Journal of Investigative Dermatology,1982, 79, 16-18.
CrossRef - Hearing, V.J.; Jiménez, M.;International Journal of Biochemistry,1987, 19, 1141-1147.
CrossRef - Prota, G.;Medicinal Research Reviews, 1988, 8, 525–556.
CrossRef - Kuzumaki, T.;Matsuda, A.; Wakamatsu, K.; ItoS.; Ishikawa, K.;Experimental Cell Research, 1993, 207, 33-40.
CrossRef - del Marmol, V.;Beermann, F.;FEBS Letters, 1996, 381, 165-168.
CrossRef - Ye, Y.; Chu, J.H.; Wang, H.; Xv, H.; Chou, G.X.; Leung, A.K.M.; Fong, W.F.; Yu, Z.L.;Journal of Ethnopharmacology,2010, 132, 533-535.
CrossRef - Jackson, I.J.; Chambers, D.M.; Tsukamoto, K.; Copeland,N.G.; Gilbert, D.J.; Jenkins, N.A.; Hearing, V.J.;EMBO Journal, 1992, 11,527-535.
CrossRef - Park, S.H.; Kim, D.S.; Kim, W.G.;Ryoo, I.J.; Lee, D.H.; Huh, C.H.;Youn, S.W.;Yoo, I.D.; Park, K.C.;Cellular and Molecular Life Sciences,2004, 61, 2878-2885.
CrossRef - Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J.;EMBO Journal, 1992, 11, 519-526.
CrossRef - Matz, H.;Tur, E.;Current Problems in Dermatology,2007, 35, 78-102.
CrossRef - Yoon, W.J.; Ham, Y.M.; Yoon, H.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G.; Natural Product Communications,2013, 8,1359-1362.
- Levy, C.;Khaled, M.; Fisher, D.E.;Trends in Molecular Medicine,2006,12, 406-414.
CrossRef - Choi, J.S.;Young, H.S.;Park, J.C.;Choi, J.H.;Woo, W.S.;Archives of Pharmacal Research,1986, 9,233-236.
CrossRef - Gerlier, D.; Thomasser, N.; Journal of Immunological Methods, 1986, 94, 57-63.
CrossRef - Liu, Y.; Progress in Neuro-Psychopharmacology and Biological Psychiatry, 1996, 23, 377-395.
CrossRef - Hill, S.E.;Buffey, J.;Thody, A.J.; Oliver, I.;Bleeheb, S.S.; Mac Neil, S.;Pigment Cell Res.,1989, 2,161-166.
CrossRef - Halaban, R.; Patton, R.S.; Cheng, E.;Svedine, S.;Trombetta, E.S.; Wahl, M.L.;Ariyan, S.; Hebert, D.N.;J. Biol. Chem.,2002, 277, 14821-14828.
CrossRef - Hunt, G.,;Kyne, S.; Ito, S.; Wakamatsu, K.; Todd, C.;Thody, A.;Pigment Cell Res., 1995,8, 202-208.
CrossRef - García-Borron, J.C.; Solano, F.;Pigment Cell Research,2002, 15, 162-173.
CrossRef - Wilcox, D.E.;Porras, A.G.; Hwang, Y.T.;Lerch, K.; Winkler, M.E.; Solomon, E.I.;Journal of the American Chemical Society,1985, 107:4015-4027.
CrossRef - Gaggioli, C.;Busca, R.; Abbe, P.;Ortonne, J.P.;Ballotti, R.;Pigment Cell Research, 2003,16, 374-382.
CrossRef - Busca, R.;Ballotti, R.;Pigment Cell Research, 2000,13, 60-69.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









