Effect of the Chemical Structure of m and p N-Vinylbenzylidene of 5-Methyl-Thiazole and 1,2,4-Triazole on Antimicrobial Activity
Mokhtaria Dehar1,2*, Seghier Ould-Kada1, Zohra Fortas3 and Soulef Dib-Bellahouel3
1Laboratory of Macro molecular Physical Chemistry, Department of Chemistry, University of Oran1 Ahmed Ben Bella, BP 1524 EL-M’Naouer, Oran 31100, Algeria.
2Laboratory of Environmental Technology, Department of Physical Chemistry, National Polytechnic School of Oran, Oran 31000, Algeria.
3Laboratory of Biology of microorganisms and Biotechnology, Department of Biotechnology, University of Oran1 Ahmed Ben Bella, BP 1524 EL-M’Naouer, Oran 31100, Algeria.
Corresponding Author E-mail: deharmokhtaria@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/320431
New Schiff bases heterocyclic structures have been synthesized and evaluated for their antibacterial and antifungal properties. They were prepared by a condensation reaction of 2-amino-5-methylthiazole, 4-amino-4H-1,2,4-triazole and 3-amino-1H-1,2,4-triazole with p-vinyl benzaldehyde 1p and m-vinyl benzaldehyde 1m. The synthesized compounds were characterized by 1H-NMR, 13C-NMR, FT-IR and UV–Vis. The compounds were examined for antibacterial and antifungal activities in vitro using the disc diffusion method. Activity against two bacterial strains (gram positive bacteria and gram negative bacteria) and two fungal strains is discussed. These compounds are active against assayed bacteria (Pseudomonas aeruginosa ATCC 26883 and Staphylococcus aureus ATCC4330,) and fungal strain (Candida albicans ATTC 10231)with minimal inhibitory concentration (MIC) value of 10 µg/mL.
KEYWORDS:1,2,4-triazole; thiazole; Schiff bases; antibacterial and antifungal activity
Download this article as:| Copy the following to cite this article: Dehar M, Kada S. O, Fortas Z, Bellahouel S. D. Effect of the Chemical Structure of M and P N-Vinylbenzylidene of 5-Methyl-Thiazole and 1,2,4-Triazole on Antimicrobial Activity. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Dehar M, Kada S. O, Fortas Z, Bellahouel S. D. Effect of the Chemical Structure of M and P N-Vinylbenzylidene of 5-Methyl-Thiazole and 1,2,4-Triazole on Antimicrobial Activity. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20751 |
Introduction
The world health organization defines the antimicrobial resistance (AMR) as the ability of microbes to resist the effects of drugs aimed at destroying them [1]. Indeed, the micro-organisms including bacteria, fungus, viruses and certain parasites are not affected by the drugs used to eliminate them. The treatment becomes inefficient and the infections they cause persist [2-4]. Although the microbial resistance development of any organism is a natural phenomenon, human actions could substantially accelerate it. Actually the research in pharmaceutical chemistry has mainly targeted the synthesis of new chemical compounds. The ultimate objective of which is to have hydrolysable therapeutic supports that offer versatile physico-chemical properties that exhibit high liberation efficiency [5].
Recently, we have synthesized the isomersvia the grafting of p-vinylbenzaldehyde and m-vinylbenzaldehyde on the active ingredient. Note that the currently used isomers in the field of drug research are either the para or the meta as requested by the in vitro and in vivo pharmacological tests on the starting molecule. In this investigation we have chosen the imine function as the labile linkage for the grafting of the 1,2,4-triazole and thiazole heterocyclic. The literature reveals that Schiff bases are important intermediates for the synthesis of some bioactive compounds [6]. They have demonstrated a versatile interesting biological actions including antibacterial, antifungal and anticancer [7-9]. The Schiff bases derivatives of 1,2,4-triazole and thiazole are also associated with a variety of applications in biology, clinical and pharmacological domains [10-12].
In this paper we describe the synthesis of Schiff bases derived from 1,2,4-triazole and thiazole. The influence of the para and the meta substitution on the antibacterial and antifungal activity of the prepared Schiff bases is carried out.
Materials and Methods
2-amino-5-methylthiazole, 4-amino-4H-1,2,4-triazole, 3-amino-1H-1,2,4-triazole, 4-chloromethylstyrene (CMS 60%) and m-vinylbenzaldehyde were purchased from Sigma Aldrich. Melting points were determined by REICHERT (N°184321) apparatus and are uncorrected. IR spectra were recorded on KBr discs using a JASCON FT/IR4200 spectrophotometer. 1H-RMN and 13C-RMN spectra were recorded (in CDCl3/DMSO-d6) as a solvent on a Bruker AC spectrometer at (200, 400, 75.5) MHz using TMS as an internal standard. UV spectra in ethanol solvent were taken on a SpectroScan 80DV UV spectrophotometer.
Results
Synthesis of P-Vinylbenzaldehyde (1p)
p-vinyl benzaldehyde was synthesized by using a mixture of 4-chloromethylstyrene (CMS 60%) and hexa methylenetetramine (HMTA) according to the method described by Sommelet [13]. The final product was distilled under reduced pressure using a vacuum pump. This compound was obtained as a yellow oil;(Yield: 79%), IR (KBr) cm-1: 2825.20 and 2735.53 (OC―H), 1701.87 (C=O), 1604.48, 989.30 and 919.87 (CH2=CH), 840.31 (C―H). 1H-RMN (CDCl3) δ ppm 5.43 (d, 1H, J =10.88 Hz, CH2=CH), 5.90 (d, 1H, J =17.60 Hz, CH2=CH), 6.78 (dd, 1H, J =10.88 Hz, J = 17.60 Hz, CH2=CH), 7.54 (d, 2H, J =8.35 Hz, H-arom), 7.84 (d, 2H, J =8.27 Hz, H-arom), 9.98(s, 1H, CH=O). 13C-RMN (CDCl3) δ ppm 117.54, 126.80, 130.16, 135.72, 135.94, 143.51 (C Vinyl, Arom), 191.80 (CH=O ald). UV (Ethanol C = 10-4 mol L-1) λmax (ε, L·mol-1·cm-1) 306 (33865), 224 (13979), 204 (13970) nm.
General Procedure for the Preparation of Schiff Bases Compounds
A mixture of equal volumes of heterocyclic amine (0.02 mol), and vinyl benzaldehyde (1pand 1m) (0.02 mol) in the presence of some traces of 2,6-di-t-butyl catechol as the polymerization inhibitor, and 4–5 drops of glacial acetic acid used as reaction catalyst in 30 mL of absolute ethanol was refluxed for 3 h in water bath as shown in the Scheme 1. The resulting solution was concentrated in vacuum and cooled down in a freezer for 24 h. The precipitated product was filtered, washed with cold absolute ethanol and then dried.
N-(4-Vinylbenzylidene)-5-Methyl-Thiazol-2-Amine (2p)
yellow crystals, [Yield: 73%]; M.p. 88-90 °C; IR (KBr) cm-1: 3078.80 (C―H arom), 1609.31 (CH=N imin), 1525.42 (CH=N thiaz), 997.01, 923.73 (C=C vinyl). 1H NMR (CDCl3) δ ppm 2.49 (d, 3H, J = 1.15 Hz, CH3 thiaz), 7.35 (d, 1H, J = 1.17 Hz CH thiaz), 5.40 (d, 1H, J = 11.10 Hz, CH2=CH), 5.90 (d, 1H, J = 17.59 Hz, CH2=CH), 6.79 (dd, 1H, J= 10.91 Hz, J = 17.60 Hz, CH2=CH), 7.52 (d, 2H, J = 8.29 Hz, H-arom), 7.93 (d, 2H, J = 8.29 Hz, H-arom), 8.92 (s, 1H, CH=N imin). 13C-RMN (CDCl3) δ ppm 12.67 (-CH3 thiaz), 116.31, 126.63, 129.98, 133.14, 134.51, 136.13, 138.95 141.51 (C Vinyl, Arom, thiaz), 161.54 (C=N thiaz), 171.14 (CH=N imin). UV (Ethanol C = 10-4 mol L-1) λmax (ε, L·mol-1·cm-1) 361 (14580), 239 (9260) nm, 213 (9680).
N-(3-Vinylbenzylidene)-5-Methyl-Thiazol-2-Amine (2m)
yellow crystals, [Yield: 72%]; M.p. 74-76 °C; IR (KBr) cm-1: 3079.76 (C―H arom), 1613.16 (CH=N), 1576.25 (CH=N thiaz), 997.89, 924.70 (C=C vinyl). 1H NMR (CDCl3) δ ppm 2.40 (d, 3H, J = 1.09 Hz, CH3 thiaz), 7.26 (d, 1H, J = 1.15 Hz CH thiaz), 5.26 (d, 1H, J = 10.95 Hz, CH2=CH), 5.77 (d, 1H, J = 17.56 Hz, CH2=CH), 6.71 (dd, 1H, J=10.90 Hz, J = 17.61 Hz, CH2=CH), 7.34-7.94 (m, 4H, H-arom), 8.86 (s, 1H, CH=N imin). 13C-RMN (CDCl3) δ ppm 12.69 (-CH3 thiaz), 115.26, 127.08, 129.09, 129.29, 130.13, 133.28, 135.39; 136.96, 138.29, 138.99 (C Vinyl, Arom, thiaz), 162.05 (C=N thiaz), 170.98 (CH=N imin). UV (Ethanol C = 10-4 mol L-1) λmax (ε, L·mol-1·cm-1) 351 (11260), 244 (16880) nm, 214 (9580).
N-(4-Vinylbenzylidene)-2H-1,2,4-Triazol-2-Amine (3p)
white crystals, (Yield: 81%); M.p. 156-158 °C; IR (KBr) cm-1: 1603.52 (CH=N imin), 1557.24 (CH=N triaz), 850.45 (C―H arom). 1H NMR (CDCl3) δ ppm 5.42 (d, 1H, J =10.90 Hz, CH2=CH), 5.90 (d, 1H, J=17.6 Hz, CH2=CH), 6.77 (dd, 1H, J =10.90 Hz, J = 17.60 Hz, CH2=CH), 7.53 (d, 2H, J=8.35 Hz, H-arom), 7.81 (d, 2H, J= 8.37 Hz, H-arom), 8.57 (s, 1H, CH=N imin), 8.62 (s, 2H, CH=N triaz). 13C-RMN (CDCl3) δ ppm 116.95, 127.02, 129.09, 130.81, 135.86, 142.10 (C vinyl, arom), 138.16 (2CH=N triaz); 156.37 (CH=N imin). UV (Ethanol C = 10-4mol L-1) λmax (ε, L·mol-1·cm-1) 306 (33865), 224 (12979), 204 (13970) nm.
N-(3-Vinylbenzylidene)-2H-1,2,4-Triazol-2-Amine (3m)
white powder, (Yield: 95%); M.p. 134-136 °C; IR (KBr) cm-1: 1595 (CH=N), 1621,84 (CH=N triaz), 890, 808.99, 712.56 (C―H arom). 1H NMR (CDCl3) δ ppm 5.40 (d, 1H, J=10.90 Hz, CH2=CH), 5.80 (d, 1H, J= 17.60 Hz, CH2=CH), 6.70 (dd, 1H, J= 10.90 Hz, J= 17.60 Hz, CH2=CH), 7.43-7.89 (m, 4H, H-arom), 8.61 (s, 1H, CH=N imin), 8.65 (s, 2H, CH=N triaz); 13C-RMN (CDCl3) δ ppm 115.81, 126.27, 128.09, 129.43, 130.49, 131.82, 135.59, 138.20 (C Vinyl, Arom), 138.63 (2CH=N triaz), 156.68 (CH=N imin), UV (Ethanol C = 10-4 mol L-1) λmax (ε, L·mol-1·cm-1) 279 (20773), 245 (30761), 209 (24372) nm.
N-(4-Vinylbenzylidene)-1H-1,2,4-Triazol-3-Amine (4p)
white crystals, (Yield: 86%); M.p. 180-182°C; IR (KBr) cm-1: 3146.29 (NH triaz) 1599.66 (CH=N imin), 1522.25 (CH=N triaz), 986.41, 908.30 (C=C vinyl). 1H NMR (DMSO-d6) δ ppm 5.40 (d, 1H, J =11.49 Hz, CH2=CH), 5.99 (d, 1H, J =17.68 Hz, CH2=CH), 6.79 (dd, 1H, J =10.99 Hz, J = 17.68 Hz, CH2=CH), 7.64 (d, 2H, J =8.31 Hz, H-arom), 7.99 (d, 2H, J =8.30 Hz, H-arom), 8.34 (d, 1H, J = 0.56 Hz, CH=N triaz), 9.32 (s, 1H, CH=N imin); 14.07 (d, 1H, J = 1.27 Hz, NH triaz). 13C-RMN (DMSO-d6) δ ppm 117.18, 127.15, 130.01, 135.17, 136.46, (C vinyl, arom), 141.35 (C=N triaz); 163.89 (CH=N imin). UV (Ethanol C = 10-4 mol L-1) λmax (ε, L·mol-1·cm-1) 317 (18460), 230 (16500), 215 (13260) nm.
N-(3-Vinylbenzylidene)-1H-1,2,4-Triazol-3-Amine (4m)
white powder, (Yield: 91%); M.p. 186-188 °C; IR (KBr) cm-1: 3240.79 (NH triaz) 1589.06 (CH=N), 1482.03 (CH=N triaz), 910.23, 786.81, 710.46 (C―H, arom). 1H NMR (DMSO-d6) δ ppm 5.48 (d, 1H, J =10.83 Hz, CH2=CH), 5.96 (d, 1H, J =17.64 Hz, CH2=CH), 6.86 (dd, 1H, J =10.84 Hz, J =17.39 Hz, CH2=CH), 7.36-8.11 (m, 4H, H-arom), 8.36 (d, 1H, J = 0.73 Hz, CH=N triaz), 9.25 (s, 1H, CH=N imin); 14.09 (d, 1H, J = 0.97 Hz, NH triaz). 13C-RMN (DMSO-d6) δ ppm 116.04, 127.28, 129.05, 129.78, 130.34, 136.12, 136.41, 138.28 (C vinyl, arom), 148.03 (C=N triaz); 163.91(CH=N imin). UV (Ethanol C = 10-4 mol L-1) λmax (ε, L·mol-1·cm-1) 393 (2080), 243 (4830), 214 (3790) nm.
Antimicrobial Activities
The newly synthesized compounds were screened in vitro for their antimicrobial activity using disc diffusion method [14,15].The following strains of bacteria and fungi were used as test microorganisms: Pseudomonas aeruginosa ATTC 26883 (Gram-positive), Staphylococcus aureus ATTC 4330 (Gram-negative), Candida albicans ATTC 10231 and Aspergillus niger ATTC 10404. The synthesizedcompounds were dissolved in sterile dimethylsulfoxide (DMSO) at different concentrations of 10, 50 and 500 µg/mL for bacteria and Candida albicans ATTC 10231 and 6.6 x 107 spores /mL for Aspergillus niger ATTC 10404. The antimicrobial activity of the compound that penetrates into the agar medium by diffusion is measured. The assays are based on the use of sterile discs filter paper (6 mm diameter) impregnated with 20 μL of the compound solution to be examined and allowed to dry at room temperature. A sterile disc impregnated with DMSO is used as negative control. After incubation for 24 h at 37 °C for bacteria plates, while fungi plates are incubated for 3 days at 25 °C, all the plates were controlled for zone of growth inhibition and a diameter of these zones was measured in millimeters. All experiments were performed in triplicates.
Results and Discussion
Chemistry
The Schiff bases were synthesized by a condensation reaction of heterocyclic amine and vinylbenzaldehyde para– and meta-substituted as shown in the Scheme 1.
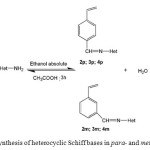 |
Scheme 1: Synthesis of heterocyclic Schiff bases in para- and meta-substituted |
H NMR Spectra
The products were identified using 1H NMR spectroscopy in chloroform and DMSO. The signals spectra were divided into four categories of protons, namely those of the vinyl, the benzene ring, the imine function and the heterocyclic groups. All the protons of the imine function CH=N of the synthesized products appear as a singlet with near signals having a slight difference between 8.57 and 9.32 ppm for 3p and 4p.The protons of the aromatic ring represent a system of AA’XX’ for the Schiff bases para-substituted but those of the meta-substituted ones form a multiplet of four protons. The chemical shifts of the vinyl groups are almost constant for all the products. A doublet at 5.42 ppm, a doublet at 5.90 ppm and a double of doublets at 6.77 ppm. However, different signals appear for the substituents of the heterocyclic rings depending on the type of their structure for example a singlet of three protons located at 2.49-2.40 ppm for the methyl group of 2m and 2prespectively as shown in the fig 1. Another singlet at 14.09-14.07ppm is assigned to (NH) of 4m and 4p.
 |
Figure 1: 1H-NMR spectrum of both isomers 2m and 2p |
C NMR Spectra
The 13C NMR spectra clearly confirm the presence of the azomethine function. It has almost the same chemical shift for the isomers containing the same heterocyclic ring. The values of 170.98-171.14 ppm are assigned to 2m and 2p structures, respectively.
FT-IR Spectra
The FT-IR spectra of the Schiff bases synthesized revealed the absence of carbonyl (C=O) stretching vibrations expected at 1701.87 cm-1. In contrast, a strong new band ranging from 1613.16 to 1599.66 cm-1 is present which is due to the azomethine (─CH=N─) linkage. The bands at 890, 808.99 and 712.56 cm-1 are assigned to (C─H) out of plane bending of the meta-substituted benzene ring. Whereas, the strong band appearing at 850.45 cm-1, is assigned to the para-substituted benzene ring.The (NH) stretching vibration of the 4p and 4m is visible at 3240.79 – 3146.29 cm-1.
UV/Vis Spectra
The UV/vis spectra of the products 2, 3 and 4 were carried out in absolute ethanol at a concentration of 10-4 mol L-1 at room temperature. All heterocyclic compounds present strong bands at λmax 361 and 279 nm. They are assigned to the electronic transition π→π* of azomethine. The para-substituted isomers exhibit a higher intensity than their respective isomers. This is due to the higher degree of molecular planarity. Shifts of the absorption bands of medium intensity between λmax 245 and 224 nm are assigned to a locally excited state of the benzal part of the molecule. These results agree with the UV spectra of aromatic and aliphatic Schiff bases reported in literature [16-18].
Antimicrobial Activities
The newly synthesized compounds were screened in vitro for their antibacterial activity against gram positive bacteria Staphylococcus aureus (ATTC 26883) and gram negative bacteria pseudomonas aeruginosa (ATTC 4330). Their antifungal activity against Candida albicans (ATTC 10231) and Aspergillus niger (ATTC 10404). The results obtained are presented in tables 1 and 2.
Table 1: Results of antibacterial evaluation of the compounds
|
Compound |
Zone of inhibition at different concentrations after 24h (mm) |
||||||
|
P. aeruginosa(ATTC 26883) |
|
S. aureus (ATTC 4330) |
|||||
|
|
10 µg/mL |
50 µg/mL |
500 µg/mL |
|
10 µg/mL |
50 µg/mL |
500 µg/mL |
|
2p |
13.5 |
15.1 |
14.1 |
14.8 |
13.8 |
10 |
|
|
2m |
14.3 |
15.2 |
15 |
12.5 |
12 |
11.5 |
|
|
3p |
12.3 |
10.8 |
12.7 |
8 |
9 |
8 |
|
|
3m |
15.3 |
15.2 |
15.5 |
9.3 |
9.8 |
8.8 |
|
|
4p |
12.7 |
14.5 |
16 |
9.1 |
10 |
8.8 |
|
|
4m |
14.7 |
15.3 |
13.7 |
10 |
10.5 |
9.8 |
|
|
DMSO |
– |
– |
– |
– |
– |
– |
|
Table 2: Results of antifungal evaluation of the compounds
|
Compound |
Zone of inhibition at different concentrations after 24h (mm) |
|
Zone of inhibition at different concentration after 3 days (mm) |
||||
|
C. alibicans (ATTC 10231) |
|
A. niger (ATTC 10404) |
|||||
|
|
10 µg/mL |
50 µg/mL |
500 µg/mL |
|
10 µg/mL |
50 µg/mL |
500 µg/mL |
|
2p |
10 |
11.5 |
10 |
9 |
– |
– |
|
|
2m |
8.1 |
13 |
12.3 |
– |
– |
– |
|
|
3p |
11.8 |
12.2 |
12.2 |
– |
– |
– |
|
|
3m |
11 |
10.2 |
11.3 |
– |
– |
– |
|
|
4p |
9.5 |
10.7 |
10.2 |
– |
– |
– |
|
|
4m |
9 |
11 |
10.2 |
– |
– |
– |
|
|
DMSO |
– |
– |
– |
– |
– |
– |
|
(-) : not active.
The majority of the synthesized products exhibit an antibacterial activity as revealed by the values of the inhibition diameters zones.
All the products tested at the three indicated concentrations are very active against Gram negative bacteria Pseudomonas aeruginosa). Generally, the products activity as tested against Staphylococcus aureus is moderate and is dependent on the type and concentration of product used. Exception to this tendency is noticed for the products 2p and 2m which showed a relatively good effect on the bacteria. The heterocyclic compounds tested in vitro against both fungal strains prove moderately active on the growth of the yeast Candida albicans. In contrast, they do not show any activity against filamentous fungiAspergillus niger except the product N-(4-vinylbenzylidene)-5-methyl-thiazol-2-amine 2p. The microbial inhibition was due to the nature of the heterocyclic thiazole which contains electron donating methyl group -CH3 present in the cycle. It has been demonstrated that the electron donating groups increase the electron density which renders the product efficient against the microorganisms [19]. In addition, the presence of the sulfur atom enhances the antimicrobial efficiency of the molecule [20].
The heterocyclic thiazoles are considered as interesting biocides [21]. The minimum inhibitory concentration (MIC) the growth of bacterial strains and that of the yeast Candida albicans is 10 µg/mL whatever the product under test. This concentration seems sufficient to inhibit the majority of the microbial strains.
The low antifungal activity and sometimes its absence is probably due to the resistance of fungal strains [22]. The results of the present investigation show that the heterocyclic compounds oriented to the para of the phenyl ring are more efficient than those oriented to themeta. This difference might originate from the fast release of the imine link CH=N of the active ingredients when the phenyl ring is para oriented.
Conclusions
In this work the new heterocyclic Schiff bases derived from 5-methyl-thiazole and 1,2,4-triazole were successfully synthesized with a high yield. The spectroscopic analysis including 1H-NMR, 13C-NMR, FT-IR and UV-Vis confirmed perfectly the expected chemical structures of these compounds. The study in vitro of antimicrobial activities showed that they exhibit antibacterial and anticandidal properties except with the fungal strains of Aspergillus niger.
Acknowledgments
Deep thanks to Dr Harrats Charef for the english review.
References
- Gilbert, P. ; McBain, A. J. Clin. Microbiol. Rev. 2003, 16, 189-208.
CrossRef - Akimitsu, N.; Hamamoto, H. ; Inoue, R i. ; Shoji, M. ; Akamine, A. ; Takemori, K –I. ; Hamasaki, N. ; Sekimizu, K. Antimicrob. Agents Chemother. 1999,43, 3042-3043.
CrossRef - McMurry, L.M.; McDermott, P.F.; Levy, S.B. Antimicrob Agents Chemother. 1999, 43, 711-713
CrossRef - Levy, S.B. Pediatr. Infect. Dis. J. 2000,19, S120-S122.
CrossRef - Benachenhou,F.; Mimouni, N.; Mederbel, Y.; Slimane,. R.K. Arabian J. Chem. 2012,5, 245-250.
CrossRef - Bertram,A.; Maulucci, N.; New, O.M.; Mohd Nor, S.M.; Pattenden, G. Org. Biomol. Chem. 2007,5, 1541-1553.
CrossRef - Posadaz, A.; Sánchez, E.; Gutiérrez, M.I.; Calderón, M.; Bertolotti, S.; Biasutti, M.A.; Garcı́a, N.A. Dyes Pigm. 2000,45, 219-228.
CrossRef - Karthikeyan,M.S.; Prasad, D.J.; Poojary, B.; Subrahmanya Bhat, K.; Holla, B.S.; Kumari, N.S. Bioorg. Med. Chem. 2006, 14, 7482-7489.
CrossRef - Schiller, D.S.; Fung, H.B. Clin. Ther. 2007, 29, 1862-1886.
CrossRef - Corouge, M.; Pol, S. Med. Maladies Infect. 2011, 41, 579-587.
CrossRef - Hu,G.; Wang, G.; Duan, N.; Wen, X.; Cao, T.; Xie, S.; Huang, W. Acta Pharm Sin B. 2012, 2, 312-317
CrossRef - Prasanna Kumar, B. N.; Mohana, K. N.; Mallesha, L. J. Fluor. Chem. 2013, 156, 15-20
CrossRef - Ferruti, P. Farmaco. Ed. Sci. 1977,32, 220
- Cruickshank,R.D. Medicinal Microbiology, 12th edition. (Churchil Livingstone, London, UK, 1975, 196-202
- Forbes,B. A. Bailey and Scott’s DiagnosticMicrobiology. 11th edition. (Mosby Inc, St. Louis, USA, 2002, 236-240
- El-Bayoumi, M. A.; El-Aasser, M.; Abdel-Halim,F. J. Am. Chem. Soc. 1971,93, 586-590.
CrossRef - Belletete, M. ; Durocher, G. C. J. C. 1982,60, 2332-2339.
- Hemmateenejad, B.; Yazdani, M.; Sharghi, H. Spectroc. Acta A. 2012,91, 198-205
CrossRef - Desai, N. C.; Bhatt, N.; Somani, H.Med. Chem. Res. 2015, 24, 258-266.
CrossRef - Siddiqui, H.L.; Zia-ur-Rehman, M.; Ahmad, N.; Weaver, G.W.; Lucas, P.D.Chem. Pharmaceut. Bull. 2007,55, 1014-1017.
CrossRef - Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Bioorg. Med. Chem.2012, 20, 1569-1583.
CrossRef - Torres, H.A.; Hachem, R.Y.; R.F.; Chemaly, Kontoyiannis, D.P.; Raad, I.I. Lancet Infect. Dis.2005, 5, 775-785.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









