Effect of Firing for Synthesis of Hydroxyapatite by Precipitation Method
Novesar Jamarun*, Zefri Azharman, Zilfa and Upita Septiani
Department of Chemistry, Andalas University, Padang, West Sumatera.
Corresponding Author E-mail: novesar@unand.ac.id
DOI : http://dx.doi.org/10.13005/ojc/320437
Hydroxyapatite (HAP) nano-powder was synthesized in a used precipitation reaction, in which calcium nitrate 4-hydrate [Ca(NO3)2.4H2O] and diammonium hydrogen phosphate [(NH4)2HPO4] were used as precursors and ammonia was used as the agent for PH adjustment. Hydroxyapatite powder has been studied at different firing temperature i.e 600, 700, 800, and 900oC to achieve high degree of crystallinity and purity. The synthesized samples were characterized by Fourier Transform Infra-red Spectroscopy (FT-IR), X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS) techniques. The result shows that, high purity of nano-hydroxyapatite powders can be obtained at 600oC, and the crystallinity and Magnification showformed rod of nano-sized grain.
KEYWORDS:Firing; Hydroxyapatite; Precipitation; Heat Treatment
Download this article as:| Copy the following to cite this article: Jamarun N, Azharman Z, Zilfa Z, Septiani U. Effect of Firing for Synthesis of Hydroxyapatite by Precipitation Method. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Jamarun N, Azharman Z, Zilfa Z, Septiani U. Effect of Firing for Synthesis of Hydroxyapatite by Precipitation Method. Orient J Chem 2016;32(4).Available from: http://www.orientjchem.org/?p=20959 |
Introduction
Hydroxyapatite (HAP), the main component of teeth and bones has received considerable attention as suitable material for bone tissue engineering because it is both biocompatible and osteoconductive1. Reported synthesis of hydroxyapatite using various reactants among others, the precursor used is calcium hydroxide and acid phosphate, calcium oxide and diammonium hydrogen phosphate, calcium nitrate 4-hydrateand diammonium hydrogen phosphate2-4.Source of calcium as precursors can be found in nature, such as corals, animal bones, shells of snails, and of limestone5-8. They have been grinded to produce smaller size. After calcining, they can turn into calcium oxide8.Techniques used are the sol-gel, hydrothermal technique, and precipitation9-12. Some synthesis techniques are used to synthesize hydroxyapatite in a way that is economical, environmentally friendly, and safe from the bilologiand simplify the complexity of the synthesis of hydroxyapatite13.
In the present work, calcium nitrate 4-hydrate [Ca(NO3)2.4H2O] from limestone is used to synthesize hydroxyapatite (HAP) with precipitation method. Firing temperature variations will be observed to determine the best temperature conditions that can form hydroxyapatite nanoparticles. The results of the synthesis of hydroxyapatite are characterized by using X-ray Difraction (XRD) to see the formation of crystals and crystal size and Scanning Electron Microscopy (SEM) to see at the morphology of the material and also Energy Dispersive X-ray Spectroscopy (EDS) to determine the value of the molar ratio of Ca/P hydroxyapatite.
Experimental
Synthesis of Hydroxyapatite, Limestone was calcinated with 900oC for 5 h, in which limestone changed the phase of calcium carbonate (CaCO3) into calcium oxide (CaO). Calcium oxide (CaO) 5.6 g were added to 100 mL of 2 M nitrate acid (HNO3) to change them into calcium nitrate hidrate (Ca(NO3)2.4H2O). The filtrate was taken, then added a solution of 100 ml 0.6 M ((NH4)2HPO4) slowly while stirring for 1 hour at 90°C at pH 11. The addition of ammonium hydroxide (NH4OH) was for pH adjustment. The solution formed was precipitated for 1 day or +15 hours until a precipitate was formed. The precipitate formed was filtered and dried at a temperature of + 110oC aiming to eliminate solvents that are still contained. The precipitate which has been dried and then grinded into powder, was then calcinated at 600, 700, 800 and 900oC for 2 hours. Nanopowder formed was then characterized.
Result and Discussion
Analysis FTIR
The results of FTIR measurements at different firing temperatures are shown in the Fig 1. Infrared spectra of HAP (Fig. 1) that informsabout the hydroxyl (OH‑) functional group by absorption band at 600, 700, 800 and 900°C, respectively at around 3453, 3571, 3571 and 3571 cm-1. FTIR spectrum is found in the P–O stretching asymmetric adsorption coming from the PO43-on 1150 – 1000 cm–1, and known as the characteristics of bands for hydroxyapatite. The absorption bands of functional groups are presence at around 1069, 1011, 1043, and 1016 cm-1.These results are consistent with those reported14.
 |
Figure 1: FTIR spectra of hydroxyapatite synthesized at (a) 600, (b) 700, (c) 800, and (d) 900oC. Click here to View table |
Analysis of XRD
Fig. 2 shows the X-ray diffraction pattern of hydroxyapatite with firing temperature variations during the firing of hidroxyapatite. While at 600°C, it has many forms of hydroxyapatite. It can be seen from the results of XRD suitability standard temperature 600°C with ICSD 16742 (Fig. 3). The presence of noise contained in the spectrum indicates the presence of amorphous hydroxyapatite while the sharp spectrum indicates the formation of hydroxyapatite crystals. From the XRD data, the size of the crystals formed can be seen by using the Scherrer equation, where a large crystal size is indicated by a sharp peak with a narrow peak width, while the small crystal size is indicated by the peak width. It is done by entering the value of FWHM (Full Width at Maximum) Scherrer equation to the known size of hydroxyapatite crystals15,16.
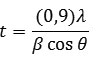
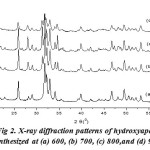 |
Figure 2: X-ray diffraction patterns of hydroxyapatite synthesized at (a) 600, (b) 700, (c) 800,and (d) 900oC
|
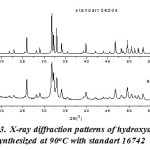 |
Figure 3: X-ray diffraction patterns of hydroxyapatite synthesized at 90oC with standart 16742 |
Where t is the particle size, λ is the wavelength used, β is the value of FWHM and θ is the Bragg diffraction angle. As for determining the specific surface area, the following formula is used:
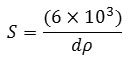
Where S is the specific surface area (m2/g), ρ is the size of the crystal and dis (3,16 g/cm3) the theoretical density of hydroxyapatite [15]. This equation can be determined based on the crystal size and specific surface areaformed at the mixing temperature variations and this can be seen in the following Table 1. Table 1 shows that the sizes of the smallest particles and the largest surface area of firing temperature are obtained at 600oC, with a particle size of 13.36 to 85.15 nm and a surface area from 22.30 to 142.08 m2/g. Thus the best firing temperature is 600oC
Table 1: The Measurements of Crystal size and Specific Surface Area Based on results of Firing Temperature Variations
|
Temperature (oC) |
Crystal size (nm) |
Specific Surface Area (m2/g) |
|
600 |
13,36 – 85,15 |
22,30 – 142,08 |
|
700 |
21,90 – 74,40 |
25,52 – 86,71 |
|
800 |
22,5 – 68,5 |
27,7 – 84,3 |
|
900 |
16,68 – 68,04 |
27,91 – 113,85 |
Analysis of SEM
Fig. 4 shows that the SEM images of the synthesis of hydroxyapatite are in the form of powder when the firing temperature of the treatment is 600°C. Magnification shows formed rod of nano-sized grains. Figure 4 shows that the measurement of particle size obtained by SEM at a temperature of 600oC is 0.09 μm – 0.71 μm
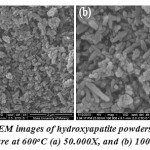 |
Figure 4: SEM images of hydroxyapatite powders of firing temperature at 600oC (a) 50.000X, and (b) 100.000X
|
Analysis of EDS
EDS analysis results indicate that the ratio of Ca/P on hydroxyapatite powder at a temperature of 600°C is more than 1.67. It indicates that the presence of other compounds such as CaO contained in the hydroxyapatite powder has been synthesized17.
Conclusion
The results of this study present an alternative method in the synthesis of hydroxyapatite namely through precipitation technique, it can be synthesized from limestone. By using the firing temperature of 600°C on calcium phosphate, nano-sized crystals are formed and a large surface area is provided. SEM results show the spherical nano-sized grains. The ratio of EDS analysis results is greater than 1.67 and it indicates the presence of other compounds are formed.
Acknowledgements
The authors thank Andalas University, Republic of Indonesia, because several parts of this work are supported by LPPM andalas University, that is under HKRGB, and Research Grant No.46//UN.16/HKRGB/LPPM/2016.
Refference
- KrishnamurithyG.;J. Heal. & Trans. Med.,2013, 16, 1-6.
- Ansari M.; Naghib S M.; Moztarzadeh F.;J. Ceramics-Silikáty., 2011, 55, 123-126.
- AdzilaS.; Sopyan I.; Hamdi M.;Int. Fed.Med. & Biol.Eng., 2011, 35, 97-101.
- Sergey V.; Dorozhkin.;Materials., 2009, 2, 1975-2045.
- ChouJ B.; Ben-Nissan.; Choi a H.; Wuhrer R and Green D.;J. Aust. Ceram. Soc., 2007, 43: 44-48.
- Xiao Ying Lü.; Yong Bin Fan.; Dachun Gu.; Wei Cui.;Key Eng. Mater.,2007, 342-343, 213-216
CrossRef - AdakM.D, K.M. Purohit., Trends Biomater. Artif. Organs., 2011, 25, 101-106 .
- Jamarun N.;Juita R and Rahayuningsih J.Research Journal of Pharmaceutical, Biological and Chemical Sciences.,2015, 6(5), 136-140
- Sari T. P.; Jamarun N.; Syukri.; Azharman Z.; Asregi A.; Orient. J. Chem., 2014, 30, 1799-1804.
CrossRef - Bingöl O. R and Durucan C.;Americ. J. Bio. Sci., 2012, 4, 50-59.
CrossRef - Eslami H.; Solati-Hashjin M.T.; reza M.; Iran. J. Pharm. Sci.,2008, 4, 127-134.
- Jamarun N.; Zefri A.; Syukri A.; Tika P. S.; Asregi A.; Elfina S.; Rasayan J. Chem., 2015, 8.
- Sobczak S.; Zygmunt Kowalski, and Zbigniew Wzorek.; Act. Bioeng. & Biomech., 2009, 11, 23-28 .
- Aziz Y.; Jamarun N.; Arief S an Nur H.; Orient. J. Chem., 2015, 31, 1099-1105.
CrossRef - Meza D.; Figueroa I A.; Flores-Morales C and Pina-Barb M C.;Rev. Mex. Fis., 2011, 57, 471–474.
- Palanivelu R A.: Rubankumar.;Int. ChemTech. Res.,2013, 5, 2965-2969.
- Abidi S A and Murtaza Q.;J. Mater. Sci. & Technol,.2014, 30, 307-310.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









