Bicyclic Diterpenoid and Phytosterol Constituents from Andrographis Paniculata (Nees)
Sonia Mol Joseph* and James T. Joseph
Department of Chemistry, Mar Ivanios College, Thiruvananthapuram 695015, Kerala, India.
Corresponding Author E-mail: sonia.ganoderma@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320455
A systematic phytochemical investigation of Andrographis paniculata led to the isolation of a bicyclic diterpenoid lactone andrographolide and a well known plant sterol β-sitosterol from the leaf extract. Andrographolide is one of the major diterpene lactone found in the pant. The structure was elucidated on the basis of spectroscopic analysis like 1H, 13C, 13C DEPT, 1H-1H COSY, FT-IR, UV-Vis and comparison with the known related compounds. It is very bitter in taste and has been reported to possess significant pharmacological activities like antioxidant, antitumor, anti HIV,cardioprotective and hepatoprotectiveactivities. Recently several studies have been conducted to investigate the pharmacological activities andrographolide proved that it is the one of the main constituent attributed for the therapeutic potential of the plant A. paniculata
KEYWORDS:Andrographis paniculata; diterpenoid; andrographolide; β-sitosterol.
Download this article as:| Copy the following to cite this article: Joseph S. M, Joseph J. T. Bicyclic Diterpenoid and Phytosterol Constituents from Andrographis Paniculata (Nees). Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Joseph S. M, Joseph J. T. Bicyclic Diterpenoid and Phytosterol Constituents from Andrographis Paniculata (Nees). Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=18942 |
Introduction
Andrographis paniculata has been used for centuries as a medicinal herb due to its unique ability to treat various ailments like jaundice, asthma, hypoglycemia, leprosy, gonorrhea, scabies, skin eruptions, hypertension, upper respiratory infections, fever, herpes and other chronic diseases1,2. In Ayurvedha more than 26 herbal formulations are mentioned from the plant revealed the widespread use for the treatment of human ailments. It is one of the commonly used plants as a popular remedy for the treatment of various liver disorders. The plant is known as the ‘king of bitters’ because it is extremely bitter in taste in every part of plant body3. On the basis of literature survey it has been observed that the aerial parts of the plants are usually used to extract the active phytoconstituents4. Previous phytochemical studies of this well studied herb showed that it is a rich source of bioactive flavonoids and diterpenoid lactones5.
A. paniculata was found to be used in traditional Indian system of medicines for lowering fever. The chief phytochemical constituent, andrographolide reported to possess antipyretic, antiulcerogenic and analgesics activity which is comparable to that of aspirin6. Different mechanisms have been proposed on anti-inflammatory activity of andrographolide involving modulating macrophage and neutrophil activity. Andrographolide is believed to be effective against various liver disorders like hepatitis; this may be responsible for the hepatoprotective avtivity of the plant7.
The purpose of this study is to identify and characterize the bioactive principles from the aerial part of A. paniculata. In this paper we report the isolation anf characterization of known compounds from the plant namely andrographolide and beta-sitosterol.
Materials and Methods
Plant materials
The fresh leaves of the plant were collected from Thiruvananthapuram district, Kerala, India in August 2014 and taxonomically identified and authenticated by Dr. Bindhu Alex, Department of Botany, Mar Ivanios College, Thiruvananthapuram.
Isolation of Andrographolide
Fresh leaves (500 g) of A. paniculata were cut into small pieces and powdered. The powdered material was first defatted with petroleum ether and then ethanol using soxhlet apparatus. Solvent evaporation under reduced pressure in a rotary evaporator yielded 2.8 and 5.6 g of petroleum ether and ethanol extracts respectively. Ethanol extract (5g) was fractionated using silica gel column chromatography (Mesh 60-120) using 100% petroleum ether, followed petroleum ether-chloroform mixture by gradient elution technique8. Different fractions obtained were pooled on the basis of comparable Rf values on TLC leading to a final three fractions (Fr.1 to Fr.3). Repeated column chromatography of Fr.1 and Fr.3 over silica gel (Mesh 100-200) yielded beta sitosterol (35 mg) and andrographolide (80 mg) respectively.
Results and Discussion
β-sitosterol was obtained as colourless needle shaped crystals from the ethanol extract of A. paniculata (Fr.1). Its molecular formula was deduced as C29H50Ofrom its EI-MS [M]+ at m/z 414. It gave positive test for steroid in the Liebermann-Burchard reaction. UV spectrum showed λmax at 202 nm indicating the presence of an olefinic group. On subjection to IR spectroscopic analysis, the observed absorption bands are appeared at3421 (OH), 2958 (CH3), 2850 (CH2), 1460, 1053 (C-O) cm-1. The compound was identified as stigmast-5-en-3β-ol (β-sitosterol) by comparison of its IR, melting point, mixed melting point and TLC data with authentic sample9,10.
Andrographolide was isolated as a crystalline solid from A. paniculata. 1H NMR spectrum of the compound was analyzed with the 1H-1H COSY data which showed signals for two tertiary methyls at δ 1.22 (3H, s) and 1.28 (3H, s), eight methylenes at δ 1.30/1.76, 1.82/1.80, 1.38/1.85, 1.44/1.91, 2.61/2.53, 4.41/4.19, 4.76/4.64 and 4.14/3.58 and five methane multiplets at δ 3.41, 1.84, 1.96, 6.54 and 4.92. The 13C NMR and DEPT spectra showed the presence of twenty carbons corresponding to two methyls at δ 21.2 and 16.2, eight methylenes at δ 40.1, 38.6, 37.7, 37.2, 36.3, 34.3, 25.8 and 24.3, five methines at δ 87.7, 81.2, 79.8, 59.6 and 55.4, four quaternary carbons including two olefins at δ 146.0, 135.2, 61.8 and 54.3 and one carbonyl carbon at δ 173.2. 1H and 13C chemical shifts in the NMR spectra of andrographolide were identical to those values reported in the literature11. Its molecular formula was deduced as C20H30O5 from its EI-MS [M]+ at m/z 350. These data proved that chemically it is 3-[2-{decahydro-6-hydroxy-5-(hydroxymethyl)-5,8α-dimethyl-2-methylene-1-naphthalenyl}ethylidene]dihydro-4-hydroxy-2(3H)-furanone [Fig. 1 & 2].
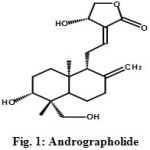 |
Figure 1: Andrographolide |
 |
Figure 2: Three dimensional model of Andrographolide |
Conclusions
The potential use of A. paniculata and its constituents in treating cancer, HIV infections and hepatitis were largely explored. Andrographolide one of its major constituent also reported to possess anticancer and immune stimulating potential12,13. Recent studies showed that andrographolide, a potent diterpenoid lactone isolated from A. paniculata inhibited the in vitro proliferation of different tumor cell lines, representing various types of human cancers. The plant extract as well as the andrographolide were also reported to induce myeloid leukemia cell differentiation in mice. These investigations reveal the therapeutic use of andrographolide and other phytoconstituents reported from A. paniculata as a potential source against various human ailments. These facts emphasize to select A. paniculata as a suitable candidate for drug development with the greatest possibility of success.
References
- Niranjan, A.; Tewari, S.K.; Lehri, A. Biological activities of Kalmegh (Andrographis paniculata Nees) and its active principles – A review. Indian. J. Nat. Prod. Resour., 2010,1, 125-135.
- Shahid, A. Andrographis paniculata: A review of pharmacological activities and clinical effects. Altern. Med. Rev., 2011, 16, 66-77.
- Mishra, S.K.; Sangwan, N.S.; Sangwan, R.S. Andrographis paniculata (Kalmegh): A review. Pharmacogn. Rev., 2007, 1, 283-298.
- Koteswara, R.Y.; Vimalamma, G.; Rao, C.V.; Tzeng Y-M. “Flavonoids and andrographolides from Andrographis paniculata.” Phytochemistry, 2004, 65, 2317–2321.
CrossRef - Li, W.; Xu, X.; Zhang, H. “Secondary metabolites from Andrographis paniculata.” Chem. Pharm. Bull., 2007, 55, 455–458.
CrossRef - Madav, S.; Tandan, S.K.; Lal, J.; Tripathi, H.C. Anti-inflammatory activity of andrographolide. Fitoterapia, 1996, 67, 452-458.
- Kapil, A.; Koul, I.B.; Banerjee, S.K.; Gupta, B.D. Antihepatotoxic effects of majorditerpenoid constituents of Andrographis paniculata. Biochem. Pharmacol., 1993, 46, 182-185.
CrossRef - Manal, M.H., Laila, A.R., Mohammed, S.A. Evaluation of antimicrobial activity of some compounds isolated from Rhamnus cathartica L. Orient. J. Chem., 2015, 31, 1133-1140.
CrossRef - Joseph, S., Janardhanan, K.K., George, V., Sabulal, B. A new epoxidic ganoderic acid and other phytoconstituents from Ganoderma lucidum. Phytochem. Lett., 2011, 4, 386-388.
CrossRef - Nish, T., Sunitha, K., Rakesh, S., Singh, C.J., Prasanth, S., Varshney, V.K. Phytochemical studies from the roots of Girardinia heterophylla. Orient. J. Chem., 2013, 29, 1143-1148.
CrossRef - Kumar, R.A.; Sridevi, K.; Kumar, N.V.; Nanduri, S.; Rajagopal, S. Anticancer anti-immunostimulatory compounds from Andrographis paniculata. J. Ethnopharm., 2004, 92, 291-295.
CrossRef - Zhao, F.; He, E.Q.; Wang, L.; Liu, K. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J. Asian Nat. Prod. Res., 2008, 10, 467-473.
CrossRef - Joseph, S. Scientific aspects of the therapeutic use of Andrographis paniculata (Kalmegh): A Review. Int. J. Pharm. Sci. Rev. Res., 2014, 27, 10-16.

This work is licensed under a Creative Commons Attribution 4.0 International License.









