An Efficient, Solvent Free One Pot Synthesis of Tetra substitue dimidazoles Catalyzed by Nanocrystalline γ-alumina
Pallavi D. Shelke1, 2, Anjali S. Rajbhoj2*, Madhav S. Nimase1, Gangaram A. Tikone1, Bhaskar H. Zaware1 and Shridhar S. Jadhav1
1Department of Chemistry, New Arts, Commerce and Science College, Ahmednagar, Affiliated to S.P. Pune University 414001 (MS), India.
2Department of Chemistry, Dr. Baba saheb Ambedkar Marathwada University, Aurangabad 431004 (MS), India.
*Corresponding Author E-mail address: anjalisrajbhoj@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320427
Article Received on :
Article Accepted on :
Article Published : 11 Aug 2016
γ-Alumina nano particles (γ-Al2O3 NPs) have been successfully synthesized by electro chemical reduction method. The aqueous solution of tetra propyl ammonium bromide was used as an electrolyte cum stabilizer. To prevent spontaneous agglomeration and control nano particles size various parameter such as current density, distance between electrodes and concentrations of electrolyte are optimized. γ-Al2O3 NPs thus synthesized were characterized by sophisticated analytical techniques including X-ray diffraction, scanning electron microscopy, energy dispersive spectro photometerand high- resolution transmission electron microscopy. These synthesized γ-Al2O3 NPs were tested used as a catalyst for one pot synthesis of tetraaryl imidazole derivatives from the cyclo dehydration and condensation of benzil, aromatic aldehyde, anilines and ammonium acetate under solvent free condition.This method has various advantages like convenient work-up procedure, environmentally benign and less reaction times along with excellent yields. Easy availability, several times recyclability, very simple isolation and eco-friendliness were some attractive features of the nano crystallineγ-Al2O3catalyst.
KEYWORDS:nano crystalline γ-alumina; electrochemical reduction method; heterogeneous catalyst; tetraaryl imidazole; solvent free
Download this article as:| Copy the following to cite this article: Shelke P. D, Rajbhoj A. S, Nimase M. S, Tikone G. A, Zaware B. H, Jadhav S. S. An Efficient, Solvent Free One Pot Synthesis of Tetra substitue dimidazoles Catalyzed by Nanocrystalline γ-alumina. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Shelke P. D, Rajbhoj A. S, Nimase M. S, Tikone G. A, Zaware B. H, Jadhav S. S. An Efficient, Solvent Free One Pot Synthesis of Tetra substitue dimidazoles Catalyzed by Nanocrystalline γ-alumina. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20585 |
Introduction
Among heterocycles, imidazoles are an important class of compounds being an active component of not only many naturally occurring products like biologically important amino acids histidine, histamine; highly significant biomolecule, vitamin B12, vitamin-H; bases in nucleic acid adenine, guanine; pilocarpinealkaloids and other alkaloids but also syntheticderivatives such as Losartan, Olmesartan, Eprosartan and Trifenagrel [1]. Imidazole and its analogues arecentre of attraction for researchers around the globe due to their diverse bioactivities. Imidazole derivatives were reported to be involved in the biosynthesis of interleukin-1 (IL-1) [2] and were also reported to function as cyclooxygenase-2 (COX-2) [3] B-Raf kinase [4], transforming growth factor β1 (TGF-β1) type 1 activin receptor-like kinase (ALK5) [5] and p38 MAP kinase [6] inhibitors. Appropriately substituted imidazoles were used as CB1 cannabinoid receptor antagonists [7], modulators of P-glycoprotein (P-gp) mediated multidrug resistance (MDR) [8] and glucagon receptors [9]. The imidazole core was reported to exhibit antiedema and anti-inflammatory [10, 11], analgesic [13], antifungal [12], antiviral [14], anthelmintic [15], antibacterial [16], antitumor [17], antitubercular [18], antibiotic, anti-ulcerative [19]. The potency and pertinence of imidazole pharmacophore is largely due to its hydrogen bond forming nature as well as its high affinity towards metals like Fe, Zn, and Mg in the protein active sites [20].
A numerous ways have been developed for the synthesis of polysubstitutedimidazoles. Condensation involving cyclodehydration of different aldehyde/substituted aldehydes, anilines/substituted anilines with benzil is an important and mostly employed method for tetrasubstituted imidazole formation in organic synthesis. The various types of bulk catalysts, such as DABCO [21], HClO4-SiO2 [22], molecular I2 [23], heteropoly acids [24], InCl3.3H2O [25], PEG-400 [26], ZrCl4 [27], K5CoW12O40.3H2O [28], silica gel or zeolite HY [29], BF3.SiO2 [30], silica bonded propyl piperazine N-sulfamic acid (SBPPSA) [31] and silica gel / NaHSO4 [32], were employed for synthetic purpose. All these catalyst / methods suffered from disadvantages like, moisture sensitive, expensive, toxic catalysts, volatile organic solvents, painstaking workup, high reaction time, higher quantities of catalysts, need of special apparatus and tedious procedures of recovery and reusability of the catalysts. Among various nano-catalysts, nanocrystalline metal oxides have found application in multi-component reactions. Some of the most frequently used metal oxides are: Fe3O4, ZnO, CuO, In2O3, TiO2, MgO, Fe2O3, ZrO2, CeO2 and Al2O3. The multi-component reactions are very lethargic in absence of catalyst. Consequently the development of a mild, simple, more efficient, cheaper and green procedure for the synthesis of highly substituted imidazole is exceedingly desirable. Solvent free reactions are becoming more popular as they are safe, nontoxic and inexpensive. The recovery of the catalyst and separation of product is easier in case of solvent free reactions than conventional routes.Use of nanoparticles as catalysts in organic transformations is one of the emerging trends currently.Literature survey showed that nanocatalysts areassociated with selectivity, reactivity and improved product yields [33-37].
In recent years, nano-catalysts have gained prominence efficiency, moisture insensitivity and greater selectivity due to their high surface area. Alumina is one of the inert biomaterial used in implants due to its biocompatible nature [38-41]. Aluminium oxide exists in number of metastable forms (γ, δ, θ, κ, ε, η, χ) and γ-Al2O3 has significant applications as a catalyst [42]. Electrochemical reduction method was firstly reported by Reetz et al [43] for the synthesis of transition metal nanoparticles.
In focus of this extensive literature survey, we have reported an efficient, solvent free synthesis of imidazoles.These multi-component reactions were carried without the assistance of any acid or base. We have synthesized γ-Al2O3 NPs by electrochemical reduction method and cyclodehydration-condensation reaction of aromatic aldehyde, anilines, ammonium acetate and benzil was successfully carried out in the presence of γ-Al2O3 NPs.
Experimental
Materials
All chemicals (upto 99% purity) were purchased from Lobachemie and Merck chemicals suppliers and liquid chemicals were purified. The purity of the chemicals was confirmed by FT-IR spectrum.Distilled water were used as a solvent.
Catalyst preparation
In the synthesis of catalyst used a sacrificial anode in the form of aluminium sheet (1 cm x 1 cm) and platinum sheet (1 cm x 1 cm) as inert cathode. These two electrodes were separated from each other by a distance 1 cm in electrolysis cell. The aqueous solution of tetra propyl ammonium bromide (TPAB 0.01M) was the electrolyte cum stabilizer. Formation of aluminium hydroxide was observed by monitoring the turbidity in solution applying constant current density 10 mA/cm2 for two h. As electrolysis process was not carried out in an inert atmosphere and therefore due to presence of dissolved moisture, the aluminium hydroxide nano particles were formed instead of pure aluminium metal nano particles. These nano particles were white in color. The intermediate product collected simply by decantation, washed with distilled water 3-4 times to remove unreacted tetra propyl ammonium bromide and dried under vacuum desiccators. This dried sample was calcined at 900 °C to convert it γ-Al2O3 NPs and stored under ambient conditions.
Catalyst characterization
The synthesized nano particles were characterized by using XRD, SEM-EDSand HRTEM techniques. The crystallinity and crystal phase of the γ-Al2O3 NPswas recorded using Bruker D8 Advance X-ray diffractometer with Cu – Kα radiation (λ = 1.5406 Å). To study morphology of γ-Al2O3 NPs SEM analysis was carried out with JEOL-JED 2300 (LA) equipment. The elemental composition of the γ-alumina nanoparticles was examined using an energy dispersive spectro photometer (EDS). The HRTEM was carried out with a FEI Model Tecnai F 30 equipment operated at 100-300kV.
General reaction procedure
Benzil (1 mmol), substituted anilines (1 mmol), benzaldehyde (1 mmol), ammonium acetate (2 mmol), and 35 mg of γ-Al2O3 NPs were mixed, stirred vigorously and heated on bare flame for 5-15 minutes. The progress of reaction was monitored by thin layer chromatography technique on aluminium TLC plate and visualization under ultraviolet (UV) light. Chloroform was added to the reaction mixture to dissolve the organic materials and γ-Al2O3nanocatalystwas filtered for recylization purpose. Chloroform was removed to separate the products. It was then recrystallized to obtain the pure compound. The products (3a-3e) were confirmed by comparison with standard data using FTIR, 1H NMR, 13C NMR and melting points. The melting points of compounds were determined with the help of digital melting / boiling point apparatus (EQ 730, Equiptronics). Infrared Spectra were recorded on Schimadzu IR-Affinity-1 FTIR spectrophotometer in cm-1 (KBr). 1H NMR and 13C NMR spectra were recorded on a BrukerAvance II 400 NMR spectrometer operated at 400 MHz and 100 MHz respectively with DMSO d6 as solvent. In NMR & CMR spectrum, chemical shift (δ) values are recorded in ppm using internal standard tetramethylsilane as an internal standard.
Result and discussion
XRD characterization
The crystallinity and crystal phase of nano particles were examined by the X-ray diffraction pattern and shown in Fig. 1. The grain or crystallinity size of nano particles is related to the diffraction peak broadening. Fig. 1 shows the XRD pattern of a synthesized sample after calcinations at 900°C for 2 h. There is a formation of single phase of well crystalline γ-Al2O3with cubic structure (JCPDS 02-1420). No peaks from impurities were observed. The (440) peak has a stronger intensity than the other peaks, indicating that the (440) planes may be preferential growth direction. The X-ray line broadening was used to calculate average particle size using Debye-Scherrer’s equation, D = kλ / βcosθ, where D is average particle size, k is shape constant, λ is wavelength, θ is diffraction angle and β is full width half maxima. The average particle size was found to be 3.93 nm.
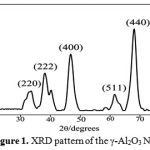 |
Figure 1: XRD pattern of the γ-Al2O3 NPs Click here to View Figure |
SEM-EDS analysis
The SEM micrograph Fig. 2(a) of γ-Al2O3 NPs showed well dispersed irregular spherical shape. The qualitative and quantitative analysis was carried outusingEDS spectrum. Results of elemental composition of γ-Al2O3 NPs are shown in Fig. 2(b). The elemental 56.72 weight % of O (68.85 atomic %) and 43.28 weight % of Al (31.15 atomic %). EDS data exhibited peaks only for Al and O which support the formation of γ-Al2O3 NPs.
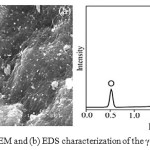 |
Figure 2:(a) SEM and (b) EDS characterization of the γ-Al2O3 NPs Click here to View Figure |
HRTEM analysis
HRTEM analysis was carried for evaluation of particle size, crystallinity and morphology of the sample. Fig. 3(a) showed that the particles were well dispersed. The HRTEM micrograph of the γ-Al2O3 NPs reveals the formation of ultra-fine nanoparticles Fig. 3(a) with the polycrystalline nature being confirmed by the ring pattern of the SAED (the insets Fig. (3a)). Lattice fringe image Fig. 3(b) exhibits the regular spacing of the lattice plane which is found to be 0.140 nm corresponding to the (440) lattice plane of the γ- phase of alumina. The atomic scale imaging Fig. 3(b) of the nanocrystallites were carried out from a small region as indicated by a circle in Fig. 3(a).
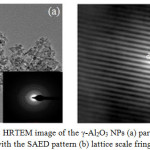 |
Figure 3: HRTEM image of the γ-Al2O3 NPs (a) particles along with the SAED pattern (b) lattice scale fringes. Click here to View Figure |
Catalytic activity
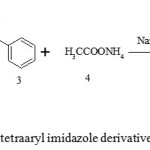 |
Scheme 1: Synthesis of tetraaryl imidazole derivatives using γ-Al2O3 NPs. Click here to View Scheme |
In present investigation, synthesis of 1,2,4,5-tetraaryl substituted imidazoles using benzil 1, substituted anilines 2, benzaldehyde 3, ammonium acetate 4 by applying γ-Al2O3 NPs as catalyst as shown in Scheme 1. All reaction was performed under dichloromethane, ethanol and solvent free condition. Physical data shown in Table 1.
Table 1: Synthesis of 1,2,4,5-tetraaryl imidzole derivatives catalyzed by γ-Al2O3 NPs
|
Producta |
-R |
Using dichloromethane |
Using ethanol |
Solvent free |
Melting point(°C) |
||||
|
Time (min) |
Yieldb (%) |
Time (min) |
Yieldb (%) |
Time (min) |
Yieldb (%) |
Found |
Reported |
||
|
3a 3b 3c 3d 3e |
-H 3-OMe 4-Me 3-Cl 4-Cl |
110 100 100 130 120 |
67 70 80 72 77 |
75 85 95 110 100 |
73 76 84 80 82 |
10 08 06 08 07 |
85 89 94 92 92 |
214-216 218-220 184-186 208-210 230-232 |
213-215 [44] New 183-185[45] 205-207[46] New |
a Reaction condition : Benzaldehyde (1 mmol), substituted anilines (1 mmol), ammonium acetate
Spectral data of compounds
1,2,4,5-Tetraphenyl-1H-imidazole
Cream crystal.1H NMR (DMSO, ppm) δ = 7.94-7.96 (m, 2H, ArH), 7.46-7.51 (m, 4H, ArH), 7.37-7.39 (m, 2H, ArH), 7.32 (m, 3H, ArH), 7.24-7.28 (m, 4H, ArH), 7.16-7.22 (m, 5H, ArH); IR-(KBr) ν = 3061 (Ar-H stretch), 1494,1600 (Aromatic C=C stretch) 1442 (C=N stretch), 1249 (Ar-N stretch)
1-(3-Chlorophenyl)-2,4,5-triphenyl-1H-imidazole
White powder. 1H NMR (DMSO, ppm) δ = 7.46-7.51 (m, 3H, ArH), 7.39-7.42(m, 3H, ArH), 7.33-7.36 (m, 7H, ArH), 7.24-7.29 (m, 5H, ArH), 7.18 (m, 1H, ArH); IR-(KBr) ν = 3050 (Ar-H stretch), 1492, 1598 (Aromatic C=C stretch), 1446, 1394 (C=N stretch), 1249, 1230, 1174 (Ar-N stretch)
1-(3-Methoxyphenyl)-2,4,5-triphenyl-1H-imidazole
Cream crystal. 1H NMR (DMSO, ppm) δ = 7.41-7.50 (m, 4H, ArH), 7.27-7.31 (m, 7H, ArH),
7.16-7.26 (m, 5H, ArH), 6.75-6.89 (m, 3H, ArH), 3.61 (s, 3H, Ar-OCH3); 13C NMR (100 MHz, DMSO) δ = 55.05, 120.49, 126.18, 126.48(s), 127.83(s), 128.01, 128.04, 128.11, 128.17(vs), 128.53, 129.16, 129.57, 130.33, 130.78, 130.85(s), 132.40, 134.30, 137.06, 137.56, 145.87, 159.38; IR-(KBr) ν = 2926 (Aliphatic C-H stretch) 3062 (Ar-H stretch), 1479,1498, 1583(Aromatic C=C stretch), 1444, 1392 (C=N stretch), 1180(Ar-N stretch)
1-(4-Chlorophenyl)-2,4,5-triphenyl-1H-imidazole
White powder. 1H NMR (DMSO, ppm) δ = 7.48-7.50 (m, 2H, ArH), 7.35-7.40 (m, 4H, ArH), 7.30-7.33 (m, 6H, ArH), 7.21-7.26 (m, 6H, ArH), 7.17 (m, 1H, ArH); 13C NMR (100 MHz, DMSO) δ = 126.28, 126.44(s), 127.85(s), 127.97(s), 128.18, 128.22(vs), 128.33, 128.99(s), 130.04(s), 130.07, 130.79, 130.90(s), 133.33, 134.13, 135.37, 137.17, 146.06; IR-(KBr) ν =3039, 3055 (Ar-H stretch), 1492,1600 (Aromatic C=C stretch), 1444, 1396 (C=N stretch), 1182 (Ar-N stretch)
1-(4-Methylphenyl)-2,4,5-triphenyl-1H-imidazole
White powder. 1H NMR (DMSO, ppm) δ = 7.44-7.59 (m, 7H, ArH), 7.34-7.40 (m, 6H, ArH), 7.28-7.33 (m, 4H, ArH), 7.15 (d, 2H, ArH), 2.25 (s, 3H, Ar-CH3); 13C NMR (100 MHz, DMSO) δ = 122.84, 123.41, 126.54, 126.85, 127.37, 127.72(s), 128.01, 128.42, 128.52(s), 128.60, 128.65, 128.83, 128.97, 129.25, 129.34, 129.58, 129.78, 129.83(s), 130.29, 131.02, 131.16, 131.53, 139.71, 143.82, 144.56, IR-(KBr) ν = 3055 (Ar-H stretch), 2964 (Aliphatic C-H stretch), 1489, 1512, 1595 (Aromatic C=C stretch), 1442 (C=N stretch), 1219 (Ar-N stretch)
The optimization of reaction condition and amount of catalyst was carried out using benzil, 3-methoxy aniline, ammonium acetate and γ-Al2O3 NPs (3c) as shown in Table 2.
Table 2: Screening of catalyst for the synthesis of 3c
|
Entry |
Catalyst amount (mg) |
Time (min) |
Yield (%) |
|
1 2 3 4 5 |
10 20 30 35 50 |
50 30 10 06 07 |
40 60 80 94 95 |
Considering the green chemistry aspect, efficient recovery and reprocess of the catalyst are highly enviable. As a result, the recovery and reusability of γ-Al2O3NPs were investigated. After completion of the reaction, the reaction mixture was dissolved in chloroform and catalyst was separated by filtration catalyst. The catalyst was washed thoroughly with ethanol and distilled water followed by activating it at 260 °C about 2 h for reusability. The reusability of the catalyst was checked for the synthesis of 1-(4-methylphenyl)-2,4,5-triphenyl-1H-imidazole (3c) and this was carried out four successive reactions giving 91, 89, 88, 84% yields of the product Table 3. The study exposed us even after four cycles, the catalyst was found to be efficient to carry out the reaction offering almost same catalytic activity.
Table 3: Studies of recyclability of γ-Al2O3 NPs in the synthesis of 3c
| Run | Fresh | 1 | 2 | 3 | 4 |
| Yield (%) | 94 | 91 | 89 | 88 | 84 |
In summary, γ-Al2O3 NPs are practical alternative to existing procedures for the synthesis of various derivatives of polysubstituted imidazole. The promising points of the present methodology were a simple, efficient and environmentally benign one pot procedure for the synthesis of 1,2,4,5-tetraaryl substituted imidazole by using catalytic amount of γ-Al2O3 NPs under thermal and solvent-free conditions. The method has salient features like mild reaction conditions, environmental compatibility, ease of isolation of product, easy separation of catalyst and easy availability of starting materials. By using above proceduregood to excellent yields of products are obtained in minimum reaction time, which might be due to Lewis acid behavior of small particle size of γ-Al2O3 NPs(with large surface area). The enhancement of the yield reveals catalyst can be used as an attractive alternative for the synthesis of many similar compounds.
Acknowledgments
The Authors are thankful to the Management of AhmednagarJilha Maratha VidyaPrasarakSamaj, Ahmednagar for constant encouragement and providing necessary research facilities. The authors are also thankful to the Director, SAIF, Punjab University, Chandigarh and National Center for Nanoscience and Nanotechnology NCNN, University of Mumbai, Mumbai.
References
- Grimmett, M. R.;Katritzky, A. R.; Rees, C. W.; Comprehensive Heterocyclic Chemistry, Eds:London, UK: pergamon Press, 1884, 374-398
- Laufer,S. A.;Zimmermann,W.;Ruff, K.J. J. Med. Chem.2004, 47, 6311-6325
CrossRef - Lange,J. H. M.;Van-Stuivenberg, H. H.;Coolen, K. K. A. C.;Adolfs,T. J. P.;McCreary,A. C.; Keizer, H. G.;Wals,H. C.;Veerman,W.;Borst,A. J. M.;de Loof, P. C. J. Med.Chem. 2005, 48, 1823-1838.
CrossRef - Takle, A. K.;Brown ,M. J. B.;Davies,S.;Dean,D. K.;Francis,G.;Gaiba,A.;Hird, A. W.; King, F.W.; Lovell, P. J.;Naylor, A. Bioorg. Med. Chem. Lett.2006, 16, 378-381.
CrossRef - Khanna,I. K.;Weier,R. M.;Yu,Y.;Xu,X. D.;Koszyk,F. J.;Collins,P. W.;Koboldt,C. M.;Veenhuizen,A. W.; Perkins, W. E.; Casler, J. J. J. Med. Chem.1997, 40, 1619-1633.
CrossRef - Lee, J. C.;Laydon, J. J.; Donnell Mc, T. F.; Gallagher, S.; Kumar, D.; Green, Nutty Mc,; Blumenthal, M. J.; Heys,R.;Landvalter,S. W.;Strickler, J. E.;McLaughlin, M. M.;Siemens, I. R.;Fischer, S. M.;Livi, J. P.;White, J. R.;Adams,J. L.;Young, P. R. Nature.1994, 372,739- 746.
CrossRef - Eyers, P. A.;Craxton,M.;Morrice,N.;Cohen, P.;Goedert, M. Chem. Biol.1998, 5, 321-328.
CrossRef - Newman, M. J.;Rodarte, J. C.;Benbatoul, K. D.;Romano, S. J.;Zhang, C.;Krane, S.; Moran, E. J.;Uyeda,R. T.;Dixon,R.;Guns,E. S. Cancer Res.2000, 60, 2964-2972.
- Laszlo De, S. E. Hacker, C.;Li, B.;Kim, D.;MacCoss, M.;Mantlo, N.;Pivnichny, J. V.; Colwell, L.;Koch, G. E.;Cascieri, M. A. Bioorg. Med. Chem. Lett. 1999, 9, 641-646.
- Breslin, H. J.;Cai, C.;Miskowski, T. A.;Coutinho, S. V.;Zhang ,S. P.; Pamela, H. Bioorg. Med. Chem. Lett.2006,16, 2505-2508.
CrossRef - Yadav, M. R.;Puntambekar, D. S.;Sarathy, K. P.;Vengurlekar, S.; GiridharR. Indian J. Chem.2006, 45B, 475-482.
- Dahiya, R. Sci. Pharm.2008,76, 217-239.
CrossRef - Khabnadi-deh, S.;Rezaei, Z.;Khalafi-Nezhad, A.;Bahrinajafi,R.;Mohamadi, R. F. Bioorg. Med. Chem. Lett.2003,13, 2863-2865.
CrossRef - Cheng, J.;Xie, J. T.;Luo, X. J. Bioorg. Med. Chem. Lett.2005,15, 267-269.
CrossRef - Kapoor, V. K.;Dubey, S.;Mahindroo, N. Indian J. Chem.2000, 39B, 27-30.
- Matysiak, J.;Niewiadomy, A.;Macik-Niewiadomy, G.;Krajewska-Kulak, E.Farmaco. 2003,58, 455-461.
CrossRef - Wang, L.;Woods, K. W.;Li, Q.;Barr, K. J.;McCroskey, R. W.;Hannick, S. M.;Gherke, L.;Credo, R.B.;Hui, Y. H.;Marsh, K.;Hannick, S. M.;Gherke, L.;Lee, J. Y.;Zielinsky- Mozng, N. ; Frost, D.;Rosenberg, S. H.;Sham, H. L. J. Med. Chem.2001, 45, 1697-1711.
CrossRef - Narayanan, S.;Vangapandu, S.;Jain, R. Bioorg. Med. Chem. Lett.2001, 11,1133-1136.
CrossRef - Ucucu, U.;Karaburun, N.G.;Iskdag, I. IL Farm.2001,56, 285-290.
CrossRef - Kuhl, O. Chem. Soc. Rev. 2007, 36, 592-607.
CrossRef - Murthy, S. N.;Madhav, B.;Nageswar, Y. V. D. Tetrahedron Lett.2010, 51, 5252-5257.
CrossRef - Kantevari, S.;Vuppalapati, S. V. N.;Biradar, D. O.;Nagarapu, L. J. Mol. Catal. A Chem. 2007, 266, 109-113.
CrossRef - Kidwai, M.;Mothsra,P.;Bansal,V.;Somvanshi,R. K.;Ethayathulla,A. S.;Dey,S.; Singh, T. P. J. Mol. Catal. A Chem.2007,265, 177-182.
CrossRef - Heravi, M.M.;Derikvand, F.;Bamoharram, F.F. J. Mol. Catal. A Chem.2007,263,112-114.
CrossRef - Sharma, S. D.;Hazarika, P.;Konwar, D. Tetrahedron Lett. 2008, 49, 2216-2220.
CrossRef - Wang,X. C.;Gong,H. P.;Quan,Z. J.;Li, L.;Ye, H. L. Chin. Chem. Lett.2009, 20, 44-47.
CrossRef - Sharma, G. V.;Jyothi,Y. P.;Lakshmi, S. Synth. Commun.2006,36, 2991-3000.
CrossRef - Nagarapu,L.;Apuri, S.;Kantevari, S. J. Mol. Catal. A Chem.2007, 266, 104-108.
CrossRef - Balalaei, S.;Arabanian, A. Green Chem.2000, 2, 274-276.
CrossRef - Sadeghi, B.;Mirjalili, B. F.;Hashemi, M. M. Tetrahedron Lett.2008, 49, 2575-2577.
CrossRef - Niknam, K.;Deris, A.;Naeimi, F.;Majleci, F. Tetrahedron Lett, 2011, 52, 4642-4645.
CrossRef - Karimi, A. R.;Alimohammadi, Z.;Azizian, J.; Mohammadi, A. A.;Mohmmadizade, M. R. Catal.Commun.2006,7, 728-732.
CrossRef - Durand, J.;Teuma, E.;Gomez, M. Eur. J .Inorg. Chem. 2008, 2008, 3577-3586.
CrossRef - Astruc, D. InorgChem, 2007,46, 1884-1894].
CrossRef - Alonso,J. F.;Yus,M. Pure Appl. Chem.2008,80,1005-1012.
CrossRef - Zhong, L. S.;Hu, J. S.;Cui, Z. M.;Wan, L. J.;Song, W. G. Chem.Mater.2007, 19,4557-4562.
CrossRef - Astruc, D. Nanopartcles Catalysis Vol. 1 Wiley-VCH, 2008.
- Hussain, S. M.;Hess, K. L.;Gearhart, J. M.;Geiss, K. T.;Schlager, J. J. Toxicol. 2005,19,975-983.
- Hanawa, T.;Kaga, M.;Itoh, Y.;Echizenya, T.;Oguchi ,H.;Ota, M. Biomaterials, 1992,13, 20-24.
CrossRef - Dey, S.;Bakthavatchalu, V.;Tseng, M. T.;Wu, P.;Florence, R. L.;Grulke, E. A.;Yokel, R. A.;Sanjit,K. D.;Yang, H S.;Chen,Y.Carcinogenesis, 2008,29,1920-1929.
CrossRef - Zielínski, P. A.;Schulz, R.;Kaliaguine, S.;van Neste, A. J. Mater. Res. 1993, 8, 2985-2992.
CrossRef - Guevara-Lara, A.;Bacaud, R.;Vrinat, M. Appl. Catal. A Gen. 2007,328, 99-108.
CrossRef - Reetz, M. T.;Helbig, W. J .Am. Chem. Soc.1994, 116, 7401-7402.
CrossRef - Davoodnia, A.; Heravi, M. M.;Safavi-Rad, Z.;Tavakoli- Hoseini, N. Synth. Commun. 2010, 7, 2588-2597.
CrossRef - Wang, X. B.;He, L.;Jian, T.Y.;Ye, S. Chin.Chem. Lett.2012, 23, 13-16.
CrossRef - Keivanloo, A.;Bakherad, M.;Inanifar, E.;Mirzzaee, M. Appl. Catal. A Gen.2013, 467, 291-300.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









