A Comprehensive Account of Spectral, NLO,NBO Analysis, Hartreefock and Density Functional Theory Studies of 1-Methyl 2, 6-Diphenyl Piperidin-4-One.
D. Sridher 1#, V. Sathya Narayana Moorthi2, J. Mallika3 and V. Kannappan4
1Department of Physics,Sri Ramakrishna Mission Vidyalaya College of Arts and Science, Coimbatore-641 020, Tamil Nadu, India.
2Department of Physics, P.S.G College of Arts and Science, Coimbatore-641 014, Tamil Nadu, India.
3Department of Chemistry, P.S.G College of Arts and Science, Coimbatore-641 014, Tamil Nadu, India.
4Department of Chemistry, Presidency College-600 005, Chennai,Tamil Nadu, India.
*Corresponding Authors E-mail: sridharsrmv@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320415
Piperidin derivatives is found to play an important role in medicinal chemistry with a wide range of pharmacological activities the piperidin ring of the title compound C18H19N1O1adopts twin chair conformation The spectroscopic property of the target compound were examined by FT-IR (4000-450cm-1), FT-RAMAN(4000-50cm-1)techniques. Theoretical calculations have been performed to obtain IR and Raman spectra of the complexes using HF and DFT methods. The vibration frequency, atomic charges, dipole moments, and several thermo dynamical parameters are reported. The charge transfer property of the molecule was verified. The first order hyper polarizability of the investigated molecule has been studied theoretically.
KEYWORDS:Spectroscopic; Pharmacological; conformation
Download this article as:| Copy the following to cite this article: Sridher D, Moorthi V. S. N, Mallika J, Kannappan V. A Comprehensive Account of Spectral, NLO,NBO Analysis, Hartreefock and Density Functional Theory Studies of 1-Methyl 2, 6-Diphenyl Piperidin-4-One. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Sridher D, Moorthi V. S. N, Mallika J, Kannappan V. A Comprehensive Account of Spectral, NLO,NBO Analysis, Hartreefock and Density Functional Theory Studies of 1-Methyl 2, 6-Diphenyl Piperidin-4-One. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20723 |
Introduction
Piperidones belongs to an important class of hetro cycles which are found to possess a variety of biological activities including cytotoxic and anticancer properties [1]. Derivatives of piperidones have attracted chemists and also biologists due to their predicted mode of interaction with little or no affinity for hydroxyl and amino groups [2,3]. CNS stimulant and recent reports suggest that compounds containing piperdin-4one moiety elicit excellent activity when aromatic substitutions are present at 2 and 6 positions. Mannich type condensation involving aromatic aldehydes and ketones have two mathylene groups, resulting in the formation of 2,6
diarylpipeidin was first reported by Noller and Baliah [4].The phenyl or para substituted phenyl substituent at C2 and C6 positions have wide range of antimicrobial activity[5].The conformational features of piperidin -4-ones are quite interesting and thought provoking. Literature report reveals that the derivatives of 2,6-diphenylpiperidin-4-ones and their stereo chemistry have been reported but however much work on N- methyl substituted in 2,6-diphenyl piperidin-4-ones have not been reported so far. This envisaged that in the present work. The 1-Methyl 2,6-diphenyl pipeidin4-one(PIP41) was synthesized and theoretical investigation was performed.
FT-Raman and FT-IR Measurement
FT-Raman spectrum of PIP41 was recorded using ND-YAG laser as excitation wavelength in the region 50-4000 cm-1 using BRUKERRFS27 stand alone spectrometer. The ND-YAG laser source operates at 1064nm line with 200mW powers. The FT-IR spectrum of the MDPO was recorded using PERKIN ELMER spectrometer in the region 4000-100cm-1. The frequencies of all sharp bands are accurate to ±1cm-1.
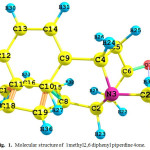 |
Figure 1: Molecular structure of 1methyl2, 6 di phenyl piperdine 4 one.
|
Computational Details
The molecules under investigation have been analysed with density functional theory (DFT) employing Beckle’s three parameter hybrid exchange functional B3LYP and HarteeFock theory (HF). In order to fit the theoretical wave numbers to the experimental, the scaling factors have been introduced by using least square optimization of the computed to the experimental data. Vibrational frequencies are scaled as 0.9067 for HF and the range of wave numbers above 1700cm-1are scaled as 0.958 and below 1700cm-1 scaled as 0.983 for B3LYP[6]. The DFT was also used to calculate the dipole moment, mean and the first static hyper polarizability (β) of the title compounds.Calculation of total molecular dipole moment μt and its components, total molecular first order hyper polarizabilityβand its components of PIP41 were made at HF and B3LYP/6-311++G level.All the calculations were performed using Gaussian 09 programme [7].
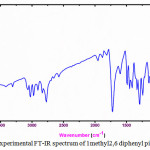 |
Figure 2: Experimental FT-IR spectrum of 1 methyl2 ,6 di phenylpiperdine 4one. |
Results and Discussion
Vibrational Analysis
Complete vibrational assignments where made. Vibrational assignments of different functional groups are analyzed in detail are discussed below. The nitrogenised heterocyclic aromatic compounds commonly shows CH stretching vibrations in the region 3010-3100 cm-1[8] The CH stretching vibrations due to pyridine ring are observed at 3140,3081,2971cm-1in IR and 3098,3010,3212cm-1 in Raman[9,10] In present investigation the experimental values shows the CH stretching vibrations at 3054,2977.2907cm-1 in Raman and in IR 3024.2978,2908cm-1 are observed
Various normal vibrations of phenyl rings are assigned according to Wilson’s numbering convention [11]. Selection rules allow the C-H stretching modes and in Raman spectrum, only the ring mode is active, exhibiting a medium polarized band at 2977 cm-1. Normal mode appear to be very weak in IR at 3054 cm-1, which is due to steric interaction that induces effective conjugation and charge carrier localization resulting in phenyl ring twisting [12]
The carbon-carbon stretching modes of the pyridine are expected in the range 1650-1100 cm-1 which is significantly influenced by the nature of the substituent [13] the six ring carbon atoms undergo coupled vibrations. The ring C=C and C-C vibrations usually occur in the region 1625-1430 cm-1The experimental frequency observed at 1600 cm‑1in Raman. The calculated frequencies in 1602 cm-1 HF and in 1358cm-1 B3LYP are assigned to this value.
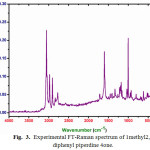 |
Figure 3: Experimental FT-Raman spectrum of 1methyl 2,6diphenylpiperdine 4one. |
Selection rule for p-di substituted phenyl ring allows five C-C stretching modes, and degenerate vibrational pair expected to be larger than which is observed as a weak band at 1495 cm-1in Raman and as a medium and at 1600 cm-1 in IR respectively. IR has contribution to mode at 1528cm-1whereas IR appears in separately as a weak band at 1408 cm-1with its counterpart appearing uniquely in Raman as a weak band at 1395 cm-1. Very strong band exists in Raman at1324 cm-1due to the ring mode.Normal vibrations are categorized as CH in-plane bending vibrations of p-di substituted phenyl rings which are given in Table 1. Allowed CH out-of-plane bending modes are very strong band in Raman at 842cm-1 and weak band in IR at818 cm-1are assigned to normal stretching modes.
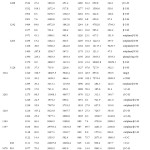 |
Table 1: Comparison of the experimental (FT-IR and FT-Raman) and theoretical harmonic wave numbers (cm-1) of 1-Methyl 2,6-diphenyl pipeidin4-one. |
Normally, in plane deformation vibration occur at higher frequencies than that of out plane vibrations [14] the lower frequency region 360-105cm-1 in Raman is due to ring deforming vibrations. The band observed at 521,502,402 cm-1in IR and 478,424cm-1 in Raman are assigned to C-C-C deformation mode in pyridine ring [15]
C=O stretching vibrations in saturated aliphatic aldehydes, ketones and acids have frequencies in range 1740-1700 cm-1. In amides the frequency is lowered to 1690 cm-1 [15] which is due to existence of resonance structures. C=O stretching band occurs at 1686 cm-1in urea[16] and is observed at 1716cm-1as a week band in Raman.1720cm-1 in IR experimental. The computed HF value1751cm-1 is assigned to this vibration.
The identification of C-N stretching vibration is difficult task, since the mixing of vibrations is possible in these regions. The C-N in plane bending vibrations is observed at 1001cm-1 in Raman. These vibrations are assigned at 1011cm-1 and1352cm-1 in computed spectrum. Increase in the value of C-N stretching is explained by shortening of C-N bond, when the data is compared with urea [15].
Frontier Molecular Orbital’s (Fmos)
The highest occupied molecular orbital’s (HOMO) and the lowest unoccupied molecular orbital’s (LUMO) are generally named as Frontier molecular orbital’s (FMO) The FMO play important role in the optical and electric properties in quantum chemistry and UV visible spectra of compounds. The HOMO-LUMO energy gap of PIP41 have been computed by using ab-initio HF/6-31G levels and the results are shown in Fig 4. It shows that the energy gap reflects the chemical activity of the molecule. LUMO as an electron acceptor and represents the ability to accept an electron; HOMO represents ability of the orbital to donate an electron.HOMO is localized on almost the whole molecule. LUMO is especially localized on the ring.The calculated energy values of HOMO are -3.31927eV, the LUMO energy value is 0.73961eV respectively. It is seen that the HOMO-LUMO gap is 4.0588eV. The energy gap between the HOMO and LUMO indicates the chemical stability of the molecules. It reveals that the molecules are soft and more reactive with chemical compounds [17, 18]
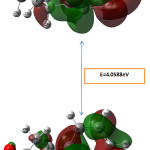 |
Figure 4: Frontier molecular orbital surfaces and energy level HOMO, LUMO of the compound. Click here to View Figure |
Mulliken Atomic Charges
Mulliken atomic charge calculation has an important role in the application of quantum chemical calculation to molecular system because of atomic charge affects dipole moment electronic structure, molecular systems. The total atomic charges of 1-Methyl 2,6-diphenyl pipeidin4-one are obtained by Mulliken population analysis shown in Table 2. The results show that substitution of methyl group in 2,6-diphenyl pipeidin4-oneleads to the redistribution of electron density. The charge distribution shows all the hydrogen atoms and carbon atoms in few sets are positively charged where as the magnitude of the carbon atomic charges are found to be both positive and negative values The charge of N positive and negative at the basis set, however the highest value 0.1928 in B3LYP method with 6-311++G(d, p) is observed. The charge of the nitrogen atom -0.0672 is lowest in HF/6-311++G(d, p). The nitrogen and oxygen atoms have negative values only in all basis sets due to electron withdrawing nature. The Mulliken charge distribution is shown in Figure5.
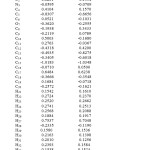 |
Table 2: Mulliken atomic charges of 1-Methyl 2,6-diphenyl pipeidin4-one |
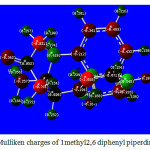 |
Figure 5: Mulliken charges of 1methyl2,6diphenylpiperdine 4one |
NBO Analysis
Accumulation of Natural Charge Population of Electrons in Core, Valence and Rydberg Orbitals
The natural population analysis performed on the electronic structure of the title molecule clearly explains the distribution of electron in various sub-shells of their atomic orbital. The accumulation of electron in the core ,valence and Rydberg sub shells of the molecule are presented in Table3. The most electro negative atoms N3, O7 and C1 have charges -0.5971,-0.67007 and -0.45658 respectively. The most electro positive atom is C6 with charge 0.58081 From the electrostatic point of view, electro negative atoms have a tendency to donate an electron whereas the electro positive atoms have a tendency to accept an electron The natural population analysis show that the distribution of 142 electrons in the title compound are distributed in sub-shells as follows:
Core 39.96791 (99.9198% of 40)
Valence 101.29463 (99.3085% of 102)
Rydberg 0.73746 (0.5193% of 142)
 |
Table 3: NBO analysis |
Core 39.96791 (99.9198% of 40)
Valence 101.29463 (99.3085% of 102)
Rydberg 0.73746 (0.5193% of 142)
The occupancies and energies of lone pair molecular orbital’s(LP)and anti bonding(BD*)molecular orbital’s of the PIP41 arepredicted at HF/6311++Glevel of the oryandis presented in Table4. The variations in occupancies and energies of the title molecule directly give the evidence for the delocalization of charge upon substitution and this leads to the variation of bond lengths.
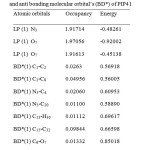 |
Table 4: Occupancy and energies of lone pair orbital’s (LP) and anti bonding molecular orbital’s(BD*) of PIP41. |
The interactions result in a loss of occupancy from the localized NBO of the idealized Lewis structure into an empty Non Lewis orbital. NBO analysis of some pharmaceutical compounds has been performed by many spectro scopists [19-21] the lone pair anti bonding interactions can be quantitatively described by the second order perturbation interaction [22-25] is shown in table 5.
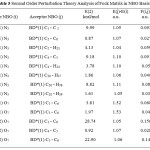 |
Table 5: Second Order Perturbation Theory Analysis of Fock Matrix in NBO Basis |
Electron Contribution in S-Type and P-Type Sub-Shells
NBO analysis of title compound is performed to estimate the delocalisation patterns of electron density (ED) from the principle occupied Lewis –type (bond or lone pair) orbitals to unoccupied non- Lewis (anti bonding or Rydberg) orbitals. The list of occupancies and energies of most interacting NBO’s along with their percentage of hybrid atomic orbitals is listed in table 6. The percentage of hybrid atomic orbitals of oxygen lone pair atom O7 and nitrogen lone pair atom N3 shows that O7 is partially contributed to both s-type and p-type sub-shells, while N3 is predominantly contributed to p-type sub-shell..In contrast ,all the anti-bonding orbital’s of the title compound are mainly contributed to p-type sub-shell, except in the BD*(1) C4-O7 orbital which shows that O7 is partially contributed to both s-type and p-type sub-shells as stated in table 6.
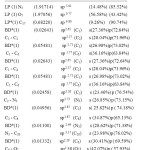 |
Table 6: Natural atomic orbital occupancies of most interacting (lone pair and anti bonding) NBOs of 1-Methyl 2,6-diphenyl pipeidin4- one |
Thermo Dynamical Properties
The values of certain thermodynamic parameters (zero point vibrational, thermal energies, specific heat capacity, rotational constants, entropy) of PIP41 at 298.15K in ground state are listed in Table 7.All thermodynamic parameters are calculated in gas phase.. The variation in zero point vibration energy (ZPVE) seems to be significant. HF method yielded higher ZPVE values than B3LYP method for the three compounds. There is only small difference in the total energy of the compound. Specific heat capacity values are computed for the molecule and these values are presented in Table 7. It is found that DFT method gave slightly higher CV values than HF method. These observations are indicative of different type of hydrogen bonding in these three molecules. Dipole moment reflects the molecular charge distribution and is given as vector in three dimensions therefore it can be used as descriptor to depict the charge distribution in the molecules. Direction of the dipole moment vector in a molecule depends on the centre of positive negative charges Dipole moments are strictly determined for neutral molecules. The computed dipole moment values are given in Table 7. It is found that HF method yielded higher dipole moment than B3LYP method. This may be due to higher atomic charges obtained by HF method.
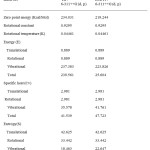 |
Table 7: The calculated thermodynamical parameters of 1-Methyl 2,6-diphenyl pipeidin4- one |
Hyperpolarizability
The complete equations for calculating, the mean polarizability α0, the anisotropy of the polarizability ∆α and the mean first polarizability βare as follows:
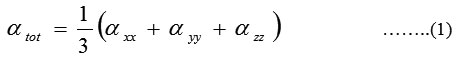
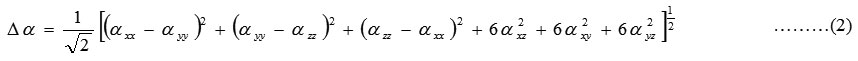
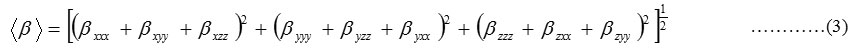
The Hyper polarizability is calculated using HF,B3LYP method on the basis of finite field approach. Calculated parameters are listed in Table8. The polariz abilities and hyper polarizability are reported in atomic units (a.u), the calculated values have been converted into electrostatic units (esu) (for α; 1 a.u = 0.1482 x10-24esu, for β; 1 a.u = 8.6393 x10-33esu). The calculated polarizability of PIP41 is 151.2×10-33esu in HF method and 139.9×10-33esu. The magnitude of the molecular hyper polarizability β, is one of the important factors in an NLO system.
 |
Table 8: Hyperpolaraizability of 1-Methyl 2,6-diphenyl pipeidin4-one |
The first order hyper polarizability is a measure of non-linear optical (NLO) effects. NLO effects arise due to interaction of incident electromagnetic fields with media (NLO materials). The effect is manifested as generation of new fields that differ in phase, frequency, amplitude or other propagation characteristics that differ from those of the incident fields [6]. NLO effects are important in providing the key functions of frequency shifting, optical modulation, optical switching, optical logic, optical memory for the emerging technologies in the area of telecommunications, signal processing and optical inter-connections [26-29].
Conclusion
The extensive vibrational analysis of 1-Methyl 2,6-diphenyl pipeidin4-one performed by HF and DFT methods with 6-311++G (d,p)basis sets.A good correlation was found between the theoretical and experimental wave numbers. The influences of acetone group, amino group to the vibrational frequencies of the title compounds were discussed. UV-visible spectra of the compound are discussed and wave lengths of maximum absorption are calculated.The UV analysis gives the electronic spectrum of PIP41 that has revealed the allowed and for bidden transitions with solvent effects.The N, O and H atoms contain negative charges and indicate that these molecules can behave as electron donors and form chelate complexes with metal ions. Especially N7 and O3 atoms favour the chelate formation as six member ring structure is possible when these two atoms are involved in the complex formation.. The dipole moment reflects the molecular charge distribution and is given as a vector in three dimensions. Mulliken charges of PIP41 at different levels were calculated and the results discussed. HOMO-LUMO energies and HOMO-LUMO energy gap are calculated as 4.0588eV. The delocalization pattern of charge and electron densities of PIP41 molecule have been explained by performing molecular orbital simulations at HF method with 6-311++G basis set. The stabilization of the structure has been identified by second order perturbation energy calculations. The calculation of the hyper polarizability gives PIP41 suitability as catalyst to increase NLO properties.
References
- Dimmock. J.R ., Arora.V. K., Wonko. S.L., Hamon. N. W., Quanil. J.W., Jia. Z.,Fang.W.D., &Lee. J. SDrug . Des Deli.,1990.,6.,183-194.
- Magee. P. NMontesano. R and Preussmann.R.,Chemical Coricinogensedited by C.E Searle. pp. American chemical society Monograph 1976., 173.,491-625.
- Mutus. B., Wagner.J.D.,TAlpas. C.J., Dimmock. J. R.,Phillips.O.A& Reid R.S., Anal.Biochem.,1989 177237-243.
- Noller CR., Baliah V., J. AM. Chem. Soc 1948.70 (11): 3853-3855
- Balasubramanian S., Aridoss G., Parthiban P., Biol Pharm Bull.2006.,29, 125-130
CrossRef - Tzeng, W. B.; Narayanan, K.; J. Mol. Strucutre (Theochem). 1908, 434, 247.
CrossRef - Gaussian 09 Program, Revision C.01, Frisch, J.; Trucks, G.W.; Schlegel, H. B.;Scuseria, G. E.; Robb, M. A.;Cheeseman, J. R.;Scalmani, G.;Barone, V.;Mennucci, B.;Petersson, A.;Nakatsuji, H.;Caricato, M.; Li, X.;Hratchian, H. P.;Izmaylov, A. F.;Bloino, J.;Zheng, G.;Sonnenberg, J. L.;Hada, M.;Ehara, M. K.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.;Kitao, O.;Nakai, O.;Vreven, T.; Montgomery, J. A.; Peralta, J. E.;Ogliaro, F.;Bearpark, M.;Heyd, J. J.; Brothers, E.;Kudin, K. N.;Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.;Raghavachari, K.; Rendell, A.;Burant, J. C.;Iyengar, S. S.;Tomasi, J.;Cossi, M.;Rega, N.;Millam, J. M.;Klene, M.; Knox, J. E.; Cross, J. B.;Bakken, V.;Adamo, C.; Jaramillo, J.;Gomperts, R.;Stratmann, R. E.;Yazyev, O.; Austin, A. J.;Cammi, R.;Pomelli, C.;Ochterski, J. W.; Martin, R. L.;Morokuma, K.;Zakrzewski, G.;Voth, G. A.; Salvador, P.; Dannenberg, J. J.;Dapprich, S.; Daniels, A. D.;Farkas, O.;Foresman, J. B.; Ortiz, J. V.;Cioslowski, J.; Fox, D. J.Gaussian, Inc., Wallingford CT, 2010.
- G.Socrates, Infrared and Raman characteristic group frequencies, third ed., Wiley, New York,2001.
- SuthanT..RajeshN.P.,MahadevanC.K.,Sajan.D.,Bhagavannarayana.G, Mater, Chem, Phys,2011.,130., 915-920.
CrossRef - Chitra.R.,, Pascal Roussel, Frederic Capet, ChitraMurli, ChoudhuryR.R.,,J.Mol.Structure, 2009.,923., 45-52
- Sudharsana.N., Keerthana.B.,Nagalakshmi.R., Krishnakumar.V.,, GuruprasadL., Mater. Chem. Phys, 2012.,134.,736-746
- Neugebauer H, Kvarnstrom C, Brabec C, Sariciftci NS, Kiebooms R, Wudl F, Luzzati S. J.Chem. Phys.1999.,110.,12108-12115.
CrossRef - Sundaraganessan N, Illkiamani S, Meganathan C & Joshua B D, SpactrochimActa A.,200767., 214-224
- BellamyL.J.,, The infra-red spectra of complex molecules, Melhuen, London, 1958
- FischerP.H.H., McDowellC.A.,,Canadian Journal of Chemistry. 1960.,38, 187-193.
- Asiri.A.M., Karabacak.M.,Kurt.M., Alamry.K.A., Spectrochim. Acta A.,201182.,444-455.
- KosarB., Albayrak.C.,,Spectrochim. ActaA.,201178.,160-167.
- ZhouZR,HongLX&ZhouZX,Indian J Pure&ApplPhys,201250.,719.
- BalachandranV,KarthickT,PerumalS&NatarajA,Indian J Pure &ApplPhys,201351.,178.
- BalasubramanianM &PadmaN,Tetrahedron,1963.,19
- Reed. A.E., &WeinholdF,J.ChemPhys,1985.,83.,1736.
- ReedA.E.,WeinstockR.B&WeinholdF,J ChemPhys,1985.,83.,735
- Reed.A.E.,&Weinhold. F.,J ChemPhys,1983.,78.,4066.
- Foster. J.P. &Wienhold.F.,J Am ChemSoc,1980.,102.,7211.
- Sun, Y.X., Hao, Q.L., Wei, W.X., Yu, Z.X., Lu, L.D., Wang, X. and Wang, Y.SJournal of Molecular Structure: THEOCHEM, .2009.,904, 74-82.
- Andraud, C., Brotin, T., Garcia, C., Pelle, F., Goldner, P., Bigot, B. and Collet, A. J of American Chem Society, 1994116, 2094-2102.
- Geskin, V.M., Lambert, C. and Bredas, J.L. J. American Chem. Society,2004125, 15651-15658.
- Nakano, M., Fujita, H., Takahata, M. and Yamaguchi, K..J. American Chem Society,2002.,124, 9648-9655.
- Sajan, D., Joe, H., Jayakumar, V.S. and Zaleski, J. J. Molecular Structure,2006785, 43-53.

This work is licensed under a Creative Commons Attribution 4.0 International License.









