The Cytotoxic Activity on T47D Breast Cancer Cell of Genistein -Standardized Ethanolic Extract of tempeh -A Fermented Product of Soybean ( Glycine Max)
Sri Hartati Yuliani, Enade Perdana Istyastono and Florentinus Dika Octa Riswanto*
1Universitas Sanata Dharma, Campus 3 Paingan, Maguwohardjo, Depok, Sleman Yogyakarta 55282, Indonesia .
Corresponding Author 's E mail : dikaocta@usd.ac.id
DOI : http://dx.doi.org/10.13005/ojc/320338
Article Received on : April 28, 2016
Article Accepted on : May 30, 2016
Article Published : 24 May 2016
As fermented soybean (Glycine max), tempeh has been reported as a good source of isoflavones, which have shown anticancer activities in various cancer cells resulted from their activity as phytoestrogens. In this article, the preparation of ethanolic extract of tempeh is presented and then followed by the exploration of its cytotoxic effect on T47D cells. The extract was subsequently standardized using genistein, the major phytoestrogen found in tempeh. The genistein concentration found in the extract of tempeh was 0.681 % (w/w). The cytotoxicity assay employing 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) resulted in IC50 values of 196.066 ± 15.956 µg/mL and 13.174 ± 0.905 µg/mL for the genistein-standardized ethanolic extract of tempeh and genistein , respectively.
KEYWORDS:Ethanolic extract; fermented soybean (tempeh); genistein; phytoestrogen
Download this article as:| Copy the following to cite this article: Yuliani S. H, Istyastono E. P, Riswanto F. D. O. The Cytotoxic Activity on T47D Breast Cancer Cell of Genistein-Standardized Ethanolic Extract of tempeh -A Fermented Product of Soybean ( Glycine Max). Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Yuliani S. H, Istyastono E. P, Riswanto F. D. O. The Cytotoxic Activity on T47D Breast Cancer Cell of Genistein-Standardized Ethanolic Extract of tempeh -A Fermented Product of Soybean ( Glycine Max). Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=16217 |
Introduction
Soybean (Glycine max (L.) Merr.) has been consumed for centuries and widely known as dietary sources for isoflavones, especially in many Asian countries1,2.Soyfoods intake was associated with lower risk of breast and prostate cancer1,3 and coronary heart disease independent on cholesterol and fracture effects1.Moreover, a high soy diet showed memory improvement, which could be associated with activities of histone deacetylases (HDACs)3,4. Similar to other phytoestrogens, the isoflavones from soybean have activities in cancer cells resulted from their activity as ligands for estrogen receptors2,5,6, a biomarker of breast cancer7–9.Notably, genistein is the most popular studied isoflavones and has been used as the reference ligand for estrogen receptors, both estrogen receptor α (ERα)and estrogen receptor β (ERβ) 10,11.
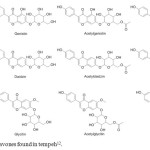 |
Figure 1: Structures of isoflavones found in tempeh12. Click here to View figure |
Tempeh, one of the fermented soy products, has been widely known as a good resource of isoflavones2,12. The structures of 12 isoflavones commonly found in tempeh are presented in Figure 11,12–14. Compared to soy flavor and other soyfoods, i.e., soy flavor, tofu, texturized vegetable protein, and soy germ, tempeh contains the highest concentration of genistein12. The activity of soyfoods in lowering cancer risk of tempeh was also associated with the activity of
genistein in inhibiting the activity of tyrosine protein kinase, an overexpressed enzyme in many cancer cells1,15,16.
The research presented in this article aimed to investigate the potency of ethanolic extract from tempeh to be developed as an herbal medicine for cancer chemoprevention. The preparation of ethanolic extract of tempeh and the subsequently standardization of the extract by analyzing the concentration of phytochemical genistein are presented in this article13,14,17–19. The cytotoxic activity on T47D cells of the genistein-standardized ethanolic extract of tempeh was evaluated and compared to genistein as the reference compound20,21.
Materials and Methods
Materials
Tempeh was collected from local market in Yogyakarta, Indonesia. Genistein and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich. Petroleum ether and ethanol for extraction, methanol, ethyl acetate, and dimethyl sulfoxide (DMSO) were purchased from Merck and distilled water was purchased from PT. Ikapharmindo Putramas (Indonesia). All solvents were at least of analytical grade or distilled before being used. The T47D cell line was cultured in Parasitology Laboratory, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Dulbecco’s Modified Eagle Media (DMEM) as medium was supplied by Gibco(R) while phosphate-buffered saline (PBS) 10% and sodium dodecyl sulfate(SDS) 10% were provided by Parasitology Laboratory, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Sodium duodecyl sulphate as stopper reagent was obtained from Merck.
Preparation of ethanolic extract of tempeh and determination of genistein content in the ethanolic extract
One kg of tempeh was blended and macerated in petroleum ether 1:1 for 40 minutes and the petroleum ether was subsequently removed. For maceration, ethanol 96% was added and the mixture was shaken at 150 rpm for 12 hours. The brown colored residue was removed and the liquid phase was evaporated using rotary evaporator for 45- 60 minutes leaving 10% extract volume compared from the initial volume. Viscous extract was dried at 50°C in an oven up toa constantweight. The ethanolic extract was then fractionated using water and ethyl acetate. The ethyl acetate was subsequently evaporated in water bath and dried until its weight was constant.
One mg of genistein as the standard was accurately weighed into a vial and dissolved in 1.0 mL ethanol. A series of genistein solutions was prepared by transferring standard genisteinsolution into 5 mL volumetric flask for each concentration and dilute to volume with ethanol to have concentrations as followed: 1.813, 3.626, 5.438, 7.250, 9.063, 10.876, 14.501, 18.126 mg/mL. Into 200 mL of ethanol, 7.3319 g of the ethanolic extract was dissolved. Ten µL of the solution was added ethanol up to 5.0 mL and was then filtered by using Millipore membrane 0.45 µm which was degassed before injection.All samples solutions were analyzed using Shimadzu LC-2010 high performance liquid chromatography (HPLC) with UV detector. The analytical column was a Phenomenex®C18 column No. 00G-4252-E0 (250 x 4.6 mm). Chromatography was carried out using methanol-redistilled water 70:10 (v/v) as the mobile phase. The flow rate of the mobile phase was maintained at 0.7 mL/min in the isocratic reverse-phase HPLC system19. The injection volume was 10 μL. Genistein content in the ethanolic extract was measured at UV 261 nm. All instruments were controlled via the software platform
LabSolution originated from Shimadzu LC-2010 HPLC.
MTT cytotoxic assay22,23
The T47D cells20 were sub cultured until confluent then 104 cells were seeded into 96-well microplate and incubated in 37oCequipped with 5% CO2 for 24 hours. DMEM medium was removed and rinsed with PBS 10% before the ethanolic extract of tempeh and genistein were dissolved in DMSO as stock. The allowed final concentration of DMSO as cytotoxic solvent used in this research was maximum 5%.The various concentrations of the ethanolic extract of tempeh and genisteinin DMEM medium were added into 96-well plate 100 μL each and incubated in 37 oC and 5% CO2 for 24 hours. Each concentration was assayed in triplicates (n=3). The DMEM medium culture was removed and rinsed by PBS 10%. Subsequently, a hundred μL DMEM containing 5 mg/mL of MTT was added into each well and incubated for 4 hours. Further, medium containing MTT was removed and 100 μL of SDS 10% was added to each well to dissolve formazan crystal. The 96-well plate was incubated for 24 hours in a dark room to avoid contact with light. Formazan crystals were then detected by ELISA reader using wavelength of 595 nm. The IC50 values were calculated using four parameters logistic regression by employing R computational statistics software version 3.1.323–25 with constrained slope value of 3.00.
Results and Discussion
The research aimed to investigate the potential of ethanolic extract from tempeh as an herbal medicine for breast cancer chemoprevention. Similar to Song and Barua14, tempeh used in this research was obtained from local market. Since there is no standardization of tempeh production, the ethanolic extract of tempeh was standardized by analyzing the concentration of genistein in the corresponding extract12–14. Genistein in soy has been accurately analyzed using reverse phase HPLC method12–14,19,26, which was therefore applied here to determine genistein concentration in the ethanolic extract.
The extraction resulted in 27 g (2.7 % w/w) of the ethanolic extract of tempeh. Isocratic chromatography was carried out using methanol-redistilled water 70:10 (v/v) as the mobile phase. The peak of genistein was found at retention time 7.350 min (Figure 2). The absorbance data versus concentration of genistein was treated using linear correlation coefficient. The following is the calibration curve equation of genistein standard resulted from the linearity test: y = 33857.0x – 3508.4 with r value of 0.9999. Employing the equation, genistein concentrations in the ethanolic extract can be calculated by examining the area under curve (AUC) resulted from the HPLC. The AUC values were 162932,169496, 164290, 166596, 164778 and 164991, which corresponded to the genistein concentrations of 245.799, 255.493, 247.805, 251.210, 248.525 and 248.840 µg/mL, respectively. Therefore, it can be concluded that the mean ± standard deviation of genistein found in the ethanolic extract of tempeh used in this research was 249.612 ± 0.003 µg/mL. Since the initial weight of the sample was 7.3319 g, the concentration of genistein in the ethanolic extract was 0.681 % (w/w). Notably, another major peak at the retention time of 6.098 min was emerged in the chromatogram of the ethanolic extract (Figure 2).
Tempeh was selected since it contains the highest concentration of genistein12,14 and as fermented product of soybean it has higher concentration of aglycon isoflavones compared to the seed14. The results showed that genistein concentration in the ethanolic extract was 0.681 % (w/w), which was approximately 7-folds higher than the total genistein concentration in tempeh, which was extracted for 2 hours using ethanol and dried using evaporator in temperature of less than 30 oC reported by Murphy et al.12. The differences in the extraction process were therefore suggested resulting in multiple higher genistein concentration in this study since the present extraction of tempeh were longer (12 hours) whereas the drying process was using higher temperature (50 oC)12,14. This result confirms that the ethanolic extract of tempeh could be a good source of aglycon isoflavones, especially genistein1,2,16. Notably, in the chromatogram presented in Figure 2, another major peak at the retention time of 6.098 min was emerged. This peak is suggested belongs to daidzein, which was reported as another major compound in tempeh that could be detected using C18 column in reverse HPLC with smaller retention time than genistein13,27. Daidzein and genistein were reported as potent ligands for estrogen receptors 6 .
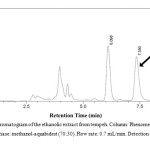 |
Figure 2: Representative chromatogram of the ethanolic extract from tempeh. Column: Phenomenex® C18 column, 250 x 4.6 mm. Mobile phase: methanol-aquabidest (70:30). Flow rate: 0.7 mL/min. Detection at 261 nm. Click here to View figure |
After the concentration of genistein in the ethanolic extract has been determined, its cytotoxic activity against T47D cell line was subsequently evaluated and compared to the cytotoxic activities of genistein11,21,28. The results are presented in Table 1. The results showed that the IC50 value of the ethanolic extract was 196.066 ± 15.956 µg/mL, while the IC50 value of genistein was 13.174 ± 0.905 µg/mL. Since genistein concentration in the ethanolic extract was only 0.681 % (w/w), other components in the extract especially other isoflavones could also play an important role in the cytotoxic activity of the ethanolic extract againstT47D cell line1,11,16.
Table 1: Effect of The Ethanolic Extract of Tempeh and Genistein on T47D Cell Viability After Incubation For 24 Hours
| Concentration (µg/mL) | Percentage of T47D viability (mean ± standard deviation) | IC50 (µg/mL)(mean ± standard deviation) |
| Ethanolic Extract of Tempeh: | ||
| 103 | 0.03 ± 0.49 | 196.066 ± 15.956a |
| 102 | 90.52 ± 1.54 | |
| 10 | 98.89 ± 5.13 | |
| 1 | 102.84 ± 5.62 | |
| 10-1 | 99.65 ± 2.45 | |
| 10-2 | 101.76 ± 5.62 | |
| 10-3 | 100.59 ± 4.38 | |
| Genistein: | ||
| 270.237 x 100 | 1.42 ± 0.49 | 13.174 ± 0.905b |
| 270.237 x 10-1 | 23.99 ± 0.71 | |
| 270.237 x 10-2 | 88.08 ± 3.75 | |
| 270.237 x 10-3 | 87.05 ± 1.90 | |
| 270.237 x 10-4 | 96.81 ± 2.80 | |
| 270.237 x 10-5 | 99.21 ± 6.06 | |
| 270.237 x 10-6 | 98.23 ± 1.17 | |
| aR2 of the logistic regression model = 0.949; bR2 of the logistic regression model = 0.990. | ||
The ethanolic extract of tempeh is therefore a good source to be developed as the active ingredients in herbal medicines for breast cancer chemoprevention. There was evidence that orally administered tamoxifen for breast cancer treatment showed severe adverse effects29,30, which could be significantly reduced by transdermal administration30,31. Therefore, development topical dosage forms containing ethanolic extract of tempeh that could deliver the aglyconeisoflavones through the skin for cancer chemoprevention is of considerable interest. Notably, although tempeh is considered as safe, further investigation to determine the safety of the ethanolic extract should be performed.32
Conclusions
The ethanolic extract of tempeh, a fermented product of soybean contains 0.681 % (w/w) genistein. The cytotoxic assay on T47D breast cancer cell of the extract and genistein as the reference using MTT method resulted in IC50 values of 196.066 ± 15.956 µg/mL and 13.174 ± 0.905 µg/mL, respectively. The ethanolic extract of tempeh could therefore serve as the active ingredients in cancer chemoprevention
Acknowledgements
We thank Rohmad Mujahid (Medicinal Plant and Traditional Medicine Research and Development Center, Surakarta, Indonesia) and Agustina Setiawati (Faculty of Pharmacy, Universitas Sanata Dharma, Yogyakarta, Indonesia) for assistance with the in vitro assay and Chrisilia Cahyani (Faculty of Pharmacy, Universitas Sanata Dharma, Yogyakarta, Indonesia) for assistance with the preparation of the ethanolic extract. This research was financially supported by the Ministry of Research Technology and Higher Education, the Government of the Indonesian Republic.
References
- Messina, M. J. Nutr.2010, 140, 2289S–2295S.
CrossRef - Messina, M.; Nagata, C.; Wu, A. H. Nutr. Cancer2006, 55, 1–12.
CrossRef - Dixon, R. A. Annu. Rev. Plant Biol.2004, 55, 225–261.
CrossRef - Istyastono, E. P.; Nurrochmad, A.; Yuniarti, N. Orient. J. Chem.2016, 32, 275–282.
CrossRef - Matsuda, H.; Shimoda, H.; Morikawa, T.; Yoshikawa, M. Bioorg. Med. Chem. Lett.2001, 11, 1839–1842.
CrossRef - Hopert, A. C.; Beyer, A.; Frank, K.; Strunck, E.; Wünsche, W.; Vollmer, G. Environ. Heal. Perspect.1998, 106, 581–586.
CrossRef - Shiau, A. K.; Barstad, D.; Loria, P. M.; Cheng, L.; Kushner, P. J.; Agard, D. A.; Greene, G. L. Cell1998, 95, 927–937.
CrossRef - Brooks, S. C.; Locke, E. R.; Soule, H. D. J. Biol. Chem.1973, 248, 6251–6253.
- Ali, S.; Coombes, R. C. J. Mammary Gland Biol. Neoplasia2000, 5, 271–281.
CrossRef - Morito, K.; Aomori, T.; Hirose, T.; Kinjo, J.; Hasegawa, J.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Biol. Pharm. Bull.2002, 25, 48–52.
CrossRef - Helferich, W. G.; Andrade, J. E.; Hoagland, M. S. Inflammopharmacology2008, 16, 219–26.
CrossRef - Murphy, P. A.; Barua, K.; Hauck, C. C. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.2002, 777, 129–138.
CrossRef - Sun, J.; Sun, B.; Han, F.; Yan, S.; Yang, H.; Akio, K. Agric. Sci. China2011, 10, 70–77.
CrossRef - Song, T.; Barua, K. Am. J. Clin. Nutr.1998, 68, 1474–1479.
CrossRef - Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. J. Biol. Chem.1987, 262, 5592–5595.
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Int. J. Mol. Sci.2015, 16, 11728–11749.
CrossRef - Yodhnu, S.; Sirikatitham, A.; Wattanapiromsakul, C. J. Chromatogr. Sci.2009, 47, 185–189.
CrossRef - Riswanto, F. D. O.; Lukitaningsih, R. R. E.; Martono, S. Indones. J. Chem.2015, 15, 9–15.
CrossRef - Vidyadhara, S.; Sasidhar, R. L. C.; Rao, B. V; Saibabu, T.; Harika, D. L. Orient. J. Chem.2016, 32, 601–607.
CrossRef - Bayala, B.; Bassole, I. H. N.; Scifo, R.; Gnoula, C.; Morel, L. Am. J. Cancer Res.2014, 4, 591–607.
- Shinde, N. V; Dhake, A. S.; Haval, K. P. Orient. J. Chem.2016, 32, 515–521.
CrossRef - Dai, Z.-J.; Ma, X.-B.; Kang, H.-F.; Gao, J.; Min, W.-L.; Guan, H.-T.; Diao, Y.; Lu, W.-F.; Wang, X.-J. Cancer Cell Int.2012, 12, 53.
CrossRef - Istyastono, E. P.; Riswanto, F. D. O.; Yuliani, S. H. Indones. J. Chem.2015, 15, 274–280.
CrossRef - Kreidenweiss, A.; Kremsner, P. G.; Mordmüller, B. Malar. J.2008, 7, 1–8.
CrossRef - R Core Team R: A Language and Environment for Statistical Computing; Vienna. http://www.r-project.org, 2015.
- Collison, M. J. AOAC Int.2008, 91, 489–500.
- Iskandar, Y. M.; Priatni, S. Indian J. Exp. Biol.2008, 8, 437–442.
- Singh, K.; Munuganti, R. S. N.; Leblanc, E.; Lin, Y. L.; Leung, E.; Lallous, N.; Butler, M.; Cherkasov, A.; Rennie, P. S. Breast Cancer Res.2015, 17, 27.
CrossRef - Cuzick, J.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A.; Forbes, J. F. Lancet Oncol.2014, 16, 67–75.
CrossRef - Lazzeroni, M.; Serrano, D.; Dunn, B. K.; Heckman-Stoddard, B. M.; Lee, O.; Khan, S.; Decensi, A. Breast Cancer Res.2012, 14, 214.
CrossRef - Sarwa, K. K.; Suresh, P. K.; Debnath, M.; Ahmad, M. Z. Curr. Drug Deliv.2013, 10, 466–476.
CrossRef - Kingsley, K.; Truong, K.; Low, E.; Hill, C. K.; Chokshi, S. B.; Phipps, D.; West, M. A.; Keiserman, M. A.; Bergman, C. J. J. Diet Suppl.2011, 8, 169–188.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









