Synthesis of Novel 2-Butyl-1H-Benzo [4, 5] Imidazo [1, 2-A] Imidazo [4, 5-E] Pyridine-5-Carbonitrile Derivatives and Evaluation of Their Anticancer Activity
Achanta Venkata Hanumantha Rao, Rayam Parsharamulu, Malthum Shankaraiah, Vadali Lakshman Rao and Anireddy Jaya Shree *
Centre for Chemical Sciences and Technology, Institute of Science and Technology, Jawaharlal Nehru Technological University Hyderabad, Kukatpally, Hyderabad-500 085, T.S, India.
Corresponding Author Email: jayashreeanireddy@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320312
Article Received on : April 25, 2016
Article Accepted on : June 01, 2016
Article Published : 26 May 2016
A series of new 2-butyl-1H-benzo[4,5]imidazo[1,2-a]imidazo[4,5-e]pyridine-5-carbonitrile derivatives were synthesized by the self cyclisation of (E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1H-imidazol-5-yl)acrylonitrile in the presence of piperidine as catalyst, which in turn were prepared by the condensation of substituted N-alkyl 2-butyl-4-chloro-1H-imidazole-5-carbaldehydes with 2-cyanomethylbenzimidazole in the presence of catalytic amount of L-proline in ethanol or by using piperidine as a base. Newly synthesized compounds which incorporate a variety of N-substituent moieties were characterized by spectral data and screened for anticancer activity against MCF-7 breast cancer cell line. The results showed that compounds 3b, 3a and 4b possess significant anti proliferative activity with IC50 values 26.59 and 27.98, 36.95 µM respectively.
KEYWORDS:Knoevenagel condensation; benzo[4,5]imidazo[1,2-a]pyridine; anticancer activity; MCF-7
Download this article as:| Copy the following to cite this article: Rao A. V. H, Parsharamulu R, Shankaraiah M, Rao V. L, Shree A. J. Synthesis of Novel 2-Butyl-1H-Benzo [4, 5] Imidazo [1, 2-A] Imidazo [4, 5-E] Pyridine-5-Carbonitrile Derivatives and Evaluation of Their Anticancer Activity. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Rao A. V. H, Parsharamulu R, Shankaraiah M, Rao V. L, Shree A. J. Synthesis of Novel 2-Butyl-1H-Benzo [4, 5] Imidazo [1, 2-A] Imidazo [4, 5-E] Pyridine-5-Carbonitrile Derivatives and Evaluation of Their Anticancer Activity. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=16755 |
Introduction
Cancer is a major health concern globally with huge number of patients suffering throughout the world. The agony and relative death rate caused by it is very high in developing countries due to the side effects associated with the known anticancer drugs in the clinical practice as well as their non affordability. Among cancer, breast cancer is the most common malignancy and the second leading cause of death among women today1. Chemotherapy is the most effective tool for cancer therapy where the use of chemotherapeutics is limited due to their undesirable side effects and also the increasing resistance to chemotherapeutic agents2. Therefore there is a need for new class of anticancer agents in treating the cancer. Among the other heterocyclic pharmacophores, imidazoles,pyridines and benzimidazoles are the most promising heterocyclic moieties, which have active templates in treating various diseases including various cancers3-6,9-11. The scaffolds imidazoles as well as pyridine are part of many successful drug candidate. Some of the anti-cancer drugs, sorfenib, nilotinib and enzalutamide showcasing these scaffolds are presented in Figure.1.
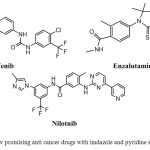 |
Figure 1: Few promising anti cancer drugs with imdazole and pyridine scaffolds. |
The strategy of conjugating two or more potent heterocyclic scaffolds in to single entity may result novel molecules with more potent activity, with same or different mode of action to treat cancer cells7, 8. In the pursuit of biologically active new chemical entities we have designed the title compounds amalgamating imidazole, benzimidzole, and pyridine scaffolds. Furthermore several reports demonstrate that the N-substitutions in both benzimidazole as well as in imidazole increase the activity12-16. Keeping in view of the above contemplation it was considered worthwhile to synthesize molecules which contain all these pharmacophores in a single entity, and we report the synthesis of newer (E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1H-imidazol-5-yl)acrylonitrile derivatives (3a-e), 2-butyl-3H-benzo[4,5]imidazo[1,2-a]imidazo[4,5-e]pyridine-5-carbonitrile derivatives (4a-e) and their preliminary anti cancer activity.
Results and Discussion
Chemistry
The synthesis of hitherto unreported title compounds 3a-e and 4a-e were accomplished in one pot as depicted in Scheme 1. Briefly, the key intermediates 3a-e were prepared in good yields by using Knoevenagel condensation17-18 in the presence of L-proline as catalyst which upon subsequent self cyclisation with piperidine under reflux to furnish the products 4a-e in excellent yields. Compounds 1b-e were synthesized by using PEG19 from 2-butyl-4-chloro-1H-imidazole-5-carbaldehyde (1a) and suitable alkylating agents.
Analytical and spectroscopic data are in full agreement with the proposed structures. A complete spectroscopic study of the compound (E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1H-imidazol-5-yl)acrylonitrile (3a) 1H NMR spectrum in DMSO-d6 showed signals at δ 0.89-0.92 (t, 3H, -CH3), 1.31-1.36 (m, 2H, -CH2), 1.62-1.7 (m, 2H, -CH2), 12.16 (s, 1H,-benzimdazole NH), 2.65-2.69 (t, 2H, -CH2), 12.98 (s, 1H, -Imidazole NH), 7.43-8.02 (m, 5H, Four of –Bz, imidazole vinyl proton) ppm. The IR spectrum of 3a showed peaks at 3015-2855 cm-1 (broad, medium,–NH), 2250 cm-1 (strong, sharp, nitrile group). Further in the mass spectra (EI) the molecular ion peak 326 due to (M+H)+ supported the formation of compound 3a. The IR spectrum of 4a (2-butyl-1H-benzo[4, 5]imidazo[1, 2-a] imidazo[4,5-e]pyridine-5-carbonitrile ) showed a peak with medium intensity at 2172 cm-1 due to nitrile group, 3015-2855 cm-1 (broad, medium,–NH). Its 1H NMR spectrum in DMSO-d6 showed signals at δ 12.11 (s, 1H, -imidazole NH), δ 6.92-7.55 (m, 5H, Four of –Bz, pyridine proton), 2.94 (t, 2H, -CH2), 2.11 (m, 2H, -CH2), 1.86 (m, 2H, -CH2) and 0.9 (t, 3H, -CH3) ppm. Mass spectrum has recorded the molecular weight of the compound 4a at 290 [M+H]+ and the fragmentation pattern is consistent with the assigned structure.
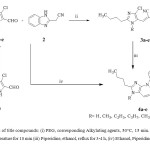 |
Scheme 1: Synthesis of title compounds: (i) PEG, corresponding Alkylating agents, 50°C, 15 min. (ii) L-Proline, ethanol, room temperature for 10 min (iii) Piperidine, ethanol, reflux for 3-4 h, (iv) Ethanol, Piperidine, reflux for 6-8 h. Click here to View scheme |
Anticancer Activity
The cytotoxicity of synthesized compounds 3a-e and 4a-e were evaluated against adenocarcinoma human breast cell line (MCF-7) using drug doxorubicin (antitumor drug) as reference standard. The percentage cell viability (IC50) for 50% cell inhibition was calculated for the compounds 3a-e and 4a-e. The anti proliferative activity of title compounds against MCF-7 cell lines in terms of IC50 is in the range of 26.59- 67.89 µM and the results are summarized in Table 3. Among the tested compounds 3b and 3a exhibited highest anticancer activity with IC50 values 26.59 and 27.98 µM respectively. Compounds 3d and 4b demonstrated good anticancer activity with IC50 values 35.42 and 36.95 µM respectively, where as compounds 3c, 3e and 4c showed moderate activity compared to other tested compounds. The result analysis revealed that the N-substituted compounds are associated with better anticancer activity. Hence it is concluded that the best anticancer activity is displayed by the compounds with the N-substitutions in imidazole moiety.
Table 1: Trypan blue assay with MCF-7 cell line for anti cancer activity in test compounds 3a-e and 4a-e
| Compounds |
Concentrations |
|||
|
1 µg/mL |
5 µg/mL |
10 µg/mL |
20 µg/mL |
|
|
3a |
208.3 c±4.5 |
212.0 c±7.93 |
193.0 c±4.0 |
184.3 c±5.03 |
|
3b |
192.0 c±4.0 |
181.3 c±2.51 |
168.6 c±3.51 |
158.3 a±3.05 |
|
3c |
181.6 c±3.78 |
169.0 b±3.0 |
160.6 b±2.08 |
149.332.51 |
|
3d |
162.0 a±3.0 |
151.33±3.05 |
142.33±3.05 |
142.66±3.05 |
|
3e |
166.0 b±4.35 |
162.0±4.0 |
154.66±3.51 |
146.0±2.64 |
|
4a |
177.0 c±2.64 |
170.0 c±2.0 |
163.3 c±3.51 |
159.3 b±1.52 |
|
4b |
191.0 c±2.0 |
186.6 c±3.05 |
182.6 c±2.51 |
178.3 c±2.08 |
|
4c |
183.3 c±5.68 |
173.3 c±2.51 |
164.3 c±2.51 |
152.66±5.68 |
|
4d |
176.0 c±2.0 |
169.0 c±2.0 |
160.6 b±2.08 |
154.0±4.35 |
|
4e |
209.6 c±3.05 |
195.0 c±2.0 |
186.0 c±3.0 |
177.6 c±3.51 |
|
Rb |
232.3 c±8.5 |
221.0 c±9.64 |
213.6 c±5.5 |
204.3 c±2.51 |
|
NC |
311.0c±7.93 |
311.6 c±8.32 |
310.2 c±4.72 |
312.6 c±3.78 |
|
PC |
— |
145.33±6.11 |
— |
— |
Note: PC= positive control (Doxorubicin hydrochloride) used as standard drug was analysed at 5 µg/mL concentration for 145.33±6.11, results mentioned as ± mean standard deviation.
NC= Negative control, P values: (a= P<0.05), (b= P<0.01), (c= P<0.001).
Table 2: MTT Assay, OD values at 550 nm in mean and standard deviation of tested compounds 3a-e and 4a-e.
|
Compounds |
MTT Assay |
|
NC |
2.21 ±0.05 |
|
VC |
1.88b ± 0.11 |
|
PC |
0.32 C ± 0.33 C |
|
Rb |
1.51 C ± 0.05 |
|
3a |
1.13 C ±0.11 |
|
3b |
1.26 C ±0.05 |
|
3c |
0.81 C ±0.05 |
|
3d |
0.61 C ±0.05 |
|
3e |
0.6 C ±0.1 |
|
4a |
1.6C ± 0.1 |
|
4b |
1.33C ±0.11 |
|
4c |
1.13C ±0.15 |
|
4d |
1.2C ±0.17 |
|
4e |
1.46C ± 0.05 |
Note: PC= Positive control, NC= Negative control, VC= Vehicle control (DMSO), P values: (a= P<0.05), (b= P<0.01), (c= P<0.001).
Table 3: Percentage (%) Cell viability of some synthesized compounds 3a-e and 4a-e against human breast Cancer MCF -7 cell line.
|
S. No. |
Test Compounds |
% Cell viability (IC50) µM |
|
1 |
3a |
27.98 |
|
2 |
3b |
26.59 |
|
3 |
3c |
44.89 |
|
4 |
3d |
35.42 |
|
5 |
3e |
60.87 |
|
6 |
4a |
51.24 |
|
7 |
4b |
36.95 |
|
8 |
4c |
67.89 |
|
9 |
4d |
65.36 |
|
10 |
4e |
48.11 |
|
11 |
VC |
87.43 |
|
12 |
PC |
14.23 |
Note: PC= Positive control, VC= Vehicle control (DMSO), IC50= Indicates the dosage levels of the compound that inhibits 50% of tumour cell proliferation.
Conclusion
In summary, synthesis and characterization of new series of benzimidazoles, imidazole linked benzimidazole analogues 3a-e and 4a-e have been described. All the tested compounds showed promising antitumor activity against MCF-7 cell line. All the derivatives evaluated showed good activity and can therefore serve as lead molecules in synthesizing new compounds which may exhibit better activity to improve further efficacy and specificity as cancer killing agents.
Materials and Methods
Chemistry
Melting points were uncorrected and are determined in open capillary tubes in sulphuric acid bath. TLC was performed on silica gel-G and spotting was done using iodine or UV light. IR spectra were recorded using Perkin-Elmer 1000 instrument in KBr phase, 1H NMR on VARIAN 400 MHz instrument and Mass spectra on Agilent-LC-MS instrument giving only M+.+1 or M+.-1 values.
General procedure for the preparation of (E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1H-imidazol-5-yl)acrylonitrile acrylonitriles derivatives (3a-e).
To a stirred solution of 2-butyl-4-chloro-1H-imidazole-5-carbaldehyde derivatives (1a-e) (10 mmol) and L-proline (15 mol %) in ethanol (10 mL), 2-cyanomethylbenzimidazole 2 (10 mmol) was added at rt. Completion of the reaction was monitored by TLC analysis. Separated solid was filtered, washed with cold ethanol (2×10 mL), dried to obtain 3a-e.
General procedure for the preparation of title compounds (4a-e).
To a solution of 3a-e (10 mmol) in ethanol (30 mL), catalytic amount of piperidine (0.2 mL) was added. The mixture was refluxed on a water bath for 3 hours till the disappearance of starting materials. After the completion of reaction, the mixture was cooled to room temperature and poured into ice cold water (50 mL). The separated solid was filtered, washed twice with cold water (2 ×20 mL) and dried to obtain 4a-e.
N-Alkylation of 2-butyl-4-chloro-1H-imidazole-5-carbaldehyde (1a) using suitable alkylating agents.
A mixture of 1a (10 mmol) in PEG-600 (20 mL) and suitable alkylating agents (11 mmol) stirred for 15 min at 50 °C. After completion of the reaction (monitored by TLC), the
mixture was poured into crushed ice. A white coloured solid separated was filtered, dried to obtain crude 1b-e. Recrystallization was done using chloroform to obtain pure product.
Cytotoxic activity studies
Trypan blue assay
In vitro experiments were performed for the synthesized compounds 3a-e and 4a-e by using trypan blue assay method on MCF-7 cell line for their anti cancer activity. Assay was performed according to the method of Lois et al20. After the measurement of assay the results were compared in both positive and negative controls.
Trypan blue solution was added to cell sample solution, where the culture was mixed to resuspend the cells. 25µL of culture sample was dispensed in a microfuge tube, to the same tube 20 µL of 0.4% trypan blue solution was added. The solution was mixed gently and transferred into a haemocytometer using micropipette where 10µL of cell culture was aspirated into the haemocytometer. The dead cells appeared in blue color where the live cells are in clear when observed under microscope (100 x magnifications).
MTT Assay
The activity assay for newly synthesized compounds were done, where the cell culture and dilution was performed according to method of Wang et al21. Cyropreserved cells were harvested, DMSO was used as vehicle control, Triton- X (1%) as positive control and synthesized compounds 3a-e and 4a-e in 0, 1, 2.5, 5 and 10 µg/mL in concentrations was used. Cell count was made using haemocytometer. Cells grown in tissue grade were plated in 96 multi well micro titer plates (104 cells / well) and were incubated at 37° C for 48 h with tested compounds(10 µg/mL) in an atmosphere of 5 % CO2. After 48 h the plates were removed and stained with MTT solution (10 µL) with gentle shaking in orbital shaker. After additional 4 h of stained plates, formazan produced wells were appeared as dark crystals. The salt crystals were solubilized using 1 % acetic acid solution and incubated over night at 37°C. Color intensity was spectrophotometrically measured by ELISA reader (Table 2). The concentration required for 50 % inhibition IC50 was calculated.
(E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1H-imidazol-5-yl) acrylonitrile (3a)
Yield 3.2g (98%), m.p. 178˚C; IR (KBr) = υmax 3015-2855 cm-1 (broad, medium,–NH-), 2250 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.89-0.92 (t, 3H, -CH3), 1.31-1.36 (m, 2H, -CH2), 1.62-1.7 (m, 2H, -CH2), 12.16 (s, 1H, -benzimdazole NH) 2.65-2.69 (t, 2H, -CH2), 12.98 (s, 1H, -Imidazole NH), 7.43-8.02 (m, 5H, Four of –Bz, imidazole vinyl proton) ppm.
(E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1-methyl-1H-imidazol-5-yl) acrylonitrile (3b)
Yield 2.9g (91%), m.p. 48˚C; IR (KBr) = υmax 3035-2955 cm-1 (broad, medium,–NH-), 2239 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.88-0.92 (t, 3H, -CH3), 1.32-1.37 (m, 2H, -CH2), 1.61-1.69 (m, 2H, -CH2), 2.63-2.8 (t, 2H, -CH2), 4.1 (s, 3H, N-CH3), 12.07 (s, 1H, -benzimidazole NH), 7.48-8.1 (m, 5H, Four of –Bz, imidazole vinyl proton) ppm.
(E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1-ethyl-1H-imidazol-5-yl)acrylonitrile (3c)
Yield 2.7g (88%), m.p. 52˚C; IR (KBr) = υmax 3015-2855 cm-1 (broad, medium,–NH-), 1650 cm-1 (strong, sharp, carbonyl group); 1H NMR (400 MHz, DMSO/TMS): δ 0.88-0.93 (t, 3H, -CH3), 1.33-1.39 (m, 2H, -CH2), 1.58-1.67 (m, 5H, -CH2 & -CH3), 2.6-2.79 (t, 2H, -CH2), 7.42-8.2 (m, 5H, Four of –Bz, imidazole vinyl proton), 4.22 (q, 2H, N-CH2), 12.04 (s,1H, Benzimdazole NH) ppm.
(E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1-propyl-1H-imidazol-5-yl)acrylonitrile (3d)
Yield 2.6g (87%), m.p. 54˚C; IR (KBr) = υmax 3015-2855 cm-1 (broad, medium,–NH-), 2230 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.87-0.95 (t, 6H, -CH3 & N-CH3), 1.34-1.42 (m, 2H, -CH2), 1.59-1.68 (m, 2H, -CH2), 2.57-2.78 (t, 2H, -CH2), 7.38-8.15 (m, 5H, Four of –Benzimidazole vinyl proton) 2.58-2.77 (t, 2H, -CH2) 1.8 (q, 2H,-CH2), 11.99 (s, 1H, Benzimdazole NH) ppm.
4(E)-2-(1H-benzo[d]imidazol-2-yl)-3-(2-butyl-4-chloro-1(methylsulfonyl)-1H-imidazol-5-yl) acrylonitrile (3e)
Yield 2.5g (88%), m.p. 48˚C; IR (KBr) = υmax 3015-2855 cm-1 (broad, medium,–NH-), 2245 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.89-0.93 (t, 3H, -CH3), 1.38-1.43 (m, 2H, -CH2), 1.57-1.64 (m, 2H, -CH2), 2.53-2.56 (t, 2H, -CH2), 7.45-8.1 (m, 5H, Four of –Bz, imdazole vinyl proton) 2.80 (s,3H,sulfonyl methyl), 11.97 (s,1H, Benzimdazole NH) ppm.
2-butyl-1H-benzo[4,5]imidazo[1,2-a]imidazo[4,5-e]pyridine-5-carbonitrile (4a)
Yield 2.2g (77%), m.p. 169˚C; IR (KBr) = υmax 3015-2855 cm-1 (broad, medium,–NH-), 2173 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.88-0.93 (t, 3H, -CH3), 1.37-1.42 (m, 2H, -CH2), 1.56-1.63 (m, 2H, -CH2), 2.51-2.57 (t, 2H, -CH2), 12.11 (s, 1H, -Imidazole NH), 7.3-7.95 (m, 5H, Four of –Bz, pyridine proton) ppm.
2-butyl-1-methyl-1H-benzo[4,5]imidazo[1,2-a]imidazo[4,5-e]pyridine-5-carbonitrile (4b)
Yield 2.14g (83%), m.p. 46˚C; IR (KBr) = υmax 3025 cm-1 (broad, medium,Ar-CH), 2240 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.87-0.91 (t, 3H, -CH3), 1.36-1.42 (m, 2H, CH2-), 1.55-1.61 (m, 2H, -CH2), 2.49-2.54 (t, 2H, -CH2), 7.28-7.91 (m, 5H, Four of –Bz, pyridine proton), 4.2 (s, 3H, N-CH3) ppm.
2-butyl-1-ethyl-1H-benzo[4,5]imidazo[1,2-a]imidazo[4,5-e]pyridine-5-carbonitrile (4c)
Yield 2.2g (90%), m.p. 49˚C; IR (KBr) = υmax 3015-2855 cm-1 (broad, medium,–NH-), 2235 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.86-0.89 (t, 3H, -CH3), 1.34-1.38 (m, 2H, -CH2), 1.53-1.58 (m, 5H, -CH2 & -CH3), 2.48-2.52 (t, 2H, -CH2), 7.24-7.88 (m, 5H, Four of –Bz,pyridine proton),4.26 (q, 2H, N-CH2) ppm.
2-butyl-1-propyl-1H-benzo[4,5]imidazo[1,2-a]imidazo[4,5-e]pyridine-5-carbonitrile (4d)
Yield 2.17g (92%), m.p. 54˚C; IR (KBr) = υmax 3005 cm-1 (broad, medium,Ar-CH), 2240 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.85-0.91 (t, 6H, -CH3 & CH3), 1.33-1.38 (m, 2H, -CH2), 1.52-1.57 (m, 2H, -CH2), 2.47-2.53 (t, 2H, -CH2), 7.14-7.85 (m, 5H, Four of –Bz, pyridine proton), 4.22 (t, 2H, N-CH2),1.78 (q, 2H, -CH2) ppm.
2-butyl-1-(methylsulfonyl)-1H-benzo[4,5]imidazo[1,2-a]imidazo[4,5-e]pyridine-5-carbonitrile (4e)
Yield 2.14g (90%), m.p. 56˚C; IR (KBr) = υmax 3015 cm-1 (broad, medium, Ar-CH), 2235 cm-1 (strong, sharp, nitrile group); 1H NMR (400 MHz, DMSO/TMS): δ 0.87-0.9 (t, 3H, -CH3), 1.34-1.37 (m, 2H, -CH2), 1.54-1.58 (m, 5H, -CH2 & -CH3), 2.49-2.53 (t, 2H, -CH2), 7.29-7.95 (m, 5H, Four of –Bz, pyridine proton), 4.26 (q, 2H, N-CH2), 2.84 (s, 3H, sulfonyl methyl) ppm.
Acknowledgements
The authors (P Rayam, S Malthum) are thankful to University Grant Commission (UGC) New Delhi, Govt. of India for financial assistance in the form of a research fellowship and one of the author (AJS) thanks DST New Delhi and TECHNICAL EDUCATION QUALITY IMPROVEMENT PROGRAMME (TEQIP) PHASE-II, India for financial support.
References
- Albrand, G.; Terret, C. Drugs Aging. 2008, 25, 35-45.
CrossRef - Federico, R.; Albanell, J.; Rovira, A.; Corominas, J. M.; Manzarbeitia, F. Semin. Diagn. Path. 2008, 25, 245-261.
CrossRef - Bansal, Y.; Sailakari, O.; Bioorg. Med. Chem. 2012, 20, 6208-6236.
CrossRef - Hranjec, M.; Starcevic, K.; Piantanida, I.; Kralj, M.; Marjanovic, M.; Hasani, M.; Westman, G.; Karminski-Zamola, G. Eur. J. Med. Chem. 2008, 43, 2877-2890.
CrossRef - Gellis, A.; Kovacic, H.; Boufatah, N.; and Vanelle, P. Eur. J. Med. Chem.2008, 43, 1858-1864.
CrossRef - Refaat, H. M. Eur. J. Med. Chem. 2010, 45, 2949-2956.
CrossRef - (a) Polkam, N.; Rayam, P.; Anireddy, J.S.; Yennam, S.; Anantaraju, H.S.; Dharmarajan S.; Perumal, Y.; Kotapalli S.S.; Ummanni R.; Balasubramanian S. Bioorg. Med. Chem. Lett.2015, 25, 1398-1402. (b) Polkam, N.; Ramaswamy, V. R.; Rayam P.; Allaka, T.R.; Anantaraju, H.S.; Dharmarajan, S.; Perumal, Y.; Gandamalla, D.; Yellu, N.R.; Balasubramanian, S.; Anireddy, J. S. Bioorg. Med. Chem. Lett. 2016, 26, 2562- 2568.
CrossRef - (a) Kuntala, N.; Telu J.R.; Banothu V.; Nallapati S.B.; Anireddy J.S.; Pal S. Med. Chem. Commun. 2015, 6, 1612-1619.
CrossRef
(b) Frere, S.; Thiery, V.; Bailly, C.; Besson, T. Tetrahedron. 2003, 59, 773-779.
CrossRef - Olgen, S. Arch. Pharm. Res. 2006, 29, 1006-1017.
CrossRef - Sheng-Yin, Z.; Yan-Wu, Y.; Hai-Quan, Z.; Yun Yue,; Mei Fan. Arch. Pharm. Res. 2011, 34, 519-526.
- Abdel motaal, E. A.; EL-Gaby, M. S. A.; Salem, M. A. Orient J Chem.2015, 31, 875-884.
- Neochorits, C. G.; Zarganes-Tzitzikas, T.; Tsoleridis, C. A. Stephanidou-Stephanatou, J.; Kontogiorgis, A. C.; Hadjipavlou-Litina, D. J.; Choli-Papadopoulou, T. Eur. J. Med. Chem. 2011, 46, 297-306.
- Ignatovich, L.; Strkova, O.; Romanovs, V.; Sleiksha, I.; Shestakova, J. P.; Lukevics, E. C. R. Chimie 2013, 16.
- Yarim, M.; Koksal, M.; Schepmann, D.; Wunsch, B. Chem. Biol. Drug Des.2011, 78, 869-875.
CrossRef - Abdel-Mohsen, H. T.; Ragab, F. A. F.; Ramla, M. M.; Diwani, H. I. Eur. J. Med. Chem.2010, 45, 2336-2344.
CrossRef - (a) Moriarty, E.; Carr, M.; Bonham, S.; Carty, P. M.; and Aldabbah, F. Eur. J. Med. Chem.2010, 45, 3762-3769. (b) Sushma, B. L.; Madhusudhan, G.; Jayashree, A.; Yeruva, K. R. Orient J Chem. 2015, 31, 2207-2212. (c) Jagadeesh, N.; Ravindrakumar, Y.; Mohanty, S.; Srinivasarao, T.; Jayashree, A. Orient J Chem. 2015, 31, 1801-1809.
- Hatamjafari, F. Orient J Chem.2013, 29, 93-95.
- Montazeri, N.; Pourshamsian, K.; Zoghi, R.; Mahjoob, S. Orient J Chem.2012, 28,103-107
CrossRef - Hanumantha Rao, A. V.; Kishore Babu, P.N.; Lakshman Rao, V.; Jaya Shree, A. Asian journal of chemistry. 2015, 27, 5, 1910-1912.
CrossRef - Lois, B. A.; Boswell, K. H.; Khwaja, T. A.; Meyer, R. B.; Robert, W. S.; Witkowski, J. T. J. Med. Chem.1978, 21, 742-745.
CrossRef - Wang, R.; Harada, S.; Mitsuya, H.; Zemlicka, J. J. Med. Chem. 2003, 46, 4799-4802.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









