Research of Porization and Adsorptions in High-Porous Adsorptive Layers of Vermiculite
K. Syrmanova1,2, M. T. Suleimenova2, N. K. Sarypbekova1, N. E. Botabaev1 and J. B. Kaldybekova1
1M. Auezov South-Kazakhstan State University, Shymkent, Kazakhstan
2Miras University Shymkent, Kazakhstan
Corresponding Author Email: syrmanova.kulash@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/320306
Article Received on : May 18, 2016
Article Accepted on : June 29, 2016
Article Published : 25 Jun 2016
Adsorption capacity of the adsorbent is dependent on the concentration of the substance in the liquid or vapor phase, its partial pressure, temperature, and the initial state of the adsorbent. At the swelling a cellular porous structure is formed, total porosity that connects with the entered number and the content of the gaseous component masses. The rheological characteristics of porous masses have the decisive effect on the porous structure. Common state for all versions of swelling is a plastic-viscous porous mass condition during their porization. The interlayer structure and inter-packet intervals may be considered as vermiculite plate micropores with dimensions of 0.3 – 1.2 nm. Vermiculite cation exchange capacity is in the range of 100-150 mEq / 100 g, i.e. from clay minerals it is one of the most interchangeable. The research results of the internal structure of adsorption layers by the adsorption isotherms means indicative of the internal surface of the porous layer is characterized by an extremely complex and developed form and can be described by means of fractal geometry. A model of the geometric structure of mica materials formed in the process of blistering during heat treatment is developed. The presented model has sufficiently general form and can be used both in the organization of systematic experimental studies of porization and adsorption in the adsorption layers of highly porous, and for the porization vermiculite optimization.
KEYWORDS:adsorption; diffusion; porosity; micropores; vermiculite; rheological properties
Download this article as:| Copy the following to cite this article: Syrmanova K, Suleimenova M. T, Sarypbekova N. K, Botabaev N. E, Kaldybekova J. B. Research of Porization and Adsorptions in High-Porous Adsorptive Layers of Vermiculite. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Syrmanova K, Suleimenova M. T, Sarypbekova N. K, Botabaev N. E, Kaldybekova J. B. Research of Porization and Adsorptions in High-Porous Adsorptive Layers of Vermiculite. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=18097 |
Introduction
In the production, transportation, processing, and emergency oil and petroleum products in large quantities enter into the industrial and waste water, and as well as into the open water, disrupting the natural biochemical processes and causing the death of flora and fauna of lakes, rivers, seas and soils . In spite of the improvements in methods of production and refining, storage and transportation of petroleum products, in the presence of various technological schemes purification of air, water and soil from oil products in general, the level of contamination is a high.
Modern wastewater treatment technologies of suspended and dissolved inorganic and organic substances are based on mechanical, physicochemical and biochemical methods, or various combinations.
The best way of the extraction of water from the dispersed and dissolved organic substances from the technological, environmental and economic points of view is the adsorptive filtration. One of the promising directions in the creation of environmentally friendly industrial production is a local treatment of liquid waste oil refinery and return to the production of purified water and valuable components.
Adsorption methods are used in deep sewage from organic substances found in small concentrations. Applications of adsorption filtration water treatment is most effective for removing water from anthropogenic element-organic compounds for the improving the quality of process water for reuse of process water, as well as protection of water bodies. This method allows for a purification step to reduce the concentration of organic compounds till 90-99%.
Among the possible technical mineral sorbents for wider use, particularly in the technology of cleaning and refining of industrial emissions, deserve special attention adsorption-active materials of natural minerals. A promising source of such materials are layer silicates, particularly vermiculite.
There are deposits of vermiculite in the Republic of Kazakhstan. The demand of the Republic of Kazakhstan in vermiculite could reach tens of thousands tons per year, due to the a wide range of applications.
In the production of porous adsorbents one of the main objectives is the creation of a high porosity that characterized by uniformity of pore volume distribution layer. These requirements determine the method and parameters of the molding process, and the type of porous structure and properties of the products. From the rheological characteristics of the binder in the production of porous materials with cellular structure of thick liquid compositions by swelling depends binder consumption, duration of mixing and molding cycle [1-3]. Ensure of the optimum processes porization and achieving the necessary geometric characteristics of the adsorbed layer requires the understanding of the formation of the layer structure and the theoretical description of this complex process.
Materials and Methods
As it is known, the adsorption is a separation method based on selective moving components of the gas or liquid phase to the surface of a solid adsorbent including in its pores. This method is effective in many cases, even those where similar separation techniques, for example using distillation, absorption or membrane systems, are ineffective or only disadvantageous. It is not surprising that the adsorption is becoming more important value in the creating of innovative technologies and in response to the growing demand in the environment protection. In addition, the improving of the adsorption methods opens new opportunities for their application.
The efficiency of the adsorption units is largely dependent on the proper selection of the adsorbent for the separation in each case. Therefore, many countries are constantly conducting research aimed at creating new adsorbents. In parallel with the development of new adsorbents conducted researches on the mechanisms of adsorption. The obtained results allow for the developing of more accurate models of adsorption processes that in turn make it possible to study adsorption phenomena in isolation, that is, eliminate the need for the researcher account the huge number of related parameters. The solution of the model equations presents no special difficulties due to the use of high-speed computers.
In most adsorption processes are carried out in a fixed bed adsorbent. In a typical process scheme uses two parallel layers with the expectation that, while one of them is in operation, the other can be subjected to regeneration.
The most important characteristics of adsorbents should be considered their adsorptive capacity, selectivity, the ability to regenerate, the kinetic parameters, suitability and cost. Moreover, it is noted that some of the rare adsorbents has optimum performance in all parameters.
The first and main parameter is the adsorption capacity. Adsorption capacity is determined by the amount of the adsorbate, adsorbent absorbed per unit mass or volume of the latter. The value of this indicator is a high, since it is crucially influenced by the amount of capital expenditures, as it determines the required amount of adsorbent and, accordingly, the size used by the adsorption towers.
For any adsorbent its adsorption capacity depends primarily on the concentration of the substance in the liquid or vapor phase, its partial pressure, temperature, and the initial state of the adsorbent. In practice, information on adsorption capacity are most often in the form of curves corresponding to fixed values of temperature, that is in the form of isotherms. This isotherm (hereinafter, this concept is discussed in detail) describes the dependence of adsorption capacity on the concentration of the adsorbate in the feed stream at a given temperature.
There are other ways of graphic expression of the adsorption capacity. Thus, the adsorption isotherm represent the relationship of the partial pressure, the condensation temperature or some other measure of the concentration of the reciprocal of the absolute temperature at a certain value of the adsorption capacity. Adsorption isobars indicate adsorption capacity as a function of temperature at a given partial pressure of a given value or other parameter concentration. The advantage of the graphs of these two types is that under certain conditions they represent a linear relationship, and this in turn facilitates calculation by interpolation and extrapolation.
The absorption capacity may also be evaluated using various numeric parameters, such as surface area, distribution of pore sizes, times, and an iodine number of the molasses number. The last two indices are used only in cases where the adsorbent acts as activated carbon.
Surface area is a relative value. Typically it is measured area of monolayer coverage of the surface of the adsorbent by substance of known density and known sizes of the molecules (for example, measures nitrogen at temperatures close to the boiling point under normal conditions). Its magnitude is generally well correlated with the values of adsorption capacity for various adsorbents can vary over a wide range from 5 to 3000 m2/g. specific ranges of surface area for the most commonly used adsorbents are given below.
The pore size distribution is a parameter which characterizes the proportion of the space within the particle occupied by the micropores (d <2,0 nm) mezapores (2.0 <= d <50.0 nm) and macropores (dp> = 50.0 nm). The values of pore sizes should be correlated with both the adsorption capacity and kinetic parameters; however, these dependencies are complex.
Fig. 1 shows the pore sizes distribution for some of the most commonly used adsorbents.
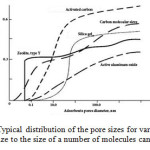 |
Figure 1: Typical distribution of the pore sizes for various adsorbents The ratio of pore size to the size of a number of molecules can be seen in Table 1. |
Table 1: Critical diameters of molecules and adsorbent pores
|
Compound |
Critical diameters of molecules, nm | Adsorbent |
Diameter of micropores, nm |
| Helium | 0.20 |
Zeolite 3А |
0.30 |
| Water | 0.27 |
Zeolite 4А |
0.40 |
| Hydrogen | 0.24 |
Zeolite 5А |
0.50 |
| Nitrogen | 0.37 |
Zeolite CaY |
0.78 |
| Oxygen | 0.34 |
Zeolite NaX |
0.80 |
| Acetylene | 0.24 |
Zeolite Mordenit |
0.70 |
| Carbon dioxide | 0.31 |
Active Al2O3 |
More 0.80 |
| Ammonia | 0.36 |
Carbon molecular sieve |
Around 0.80 |
| Methane | 0.37 |
Activated carbon |
More 0.60 |
| Propane | 0.49 |
Silica gel |
More 1.00 |
Iodine number is an approximate measure of the ability of the substance for the adsorption of small molecules that depends on the magnitude of the surface area. Molasses number, previously used only for the process control discoloration of raw cane sugar, it is used in the characterizing of the adsorption of large molecules from the liquid phases.
The ratio of the adsorption capacity of the adsorbent by one component to its capacity for adsorption of another component with a given concentration of the liquid phase is called selectivity. Generally, the lower the concentration of zero, this ratio approaches a constant value. Ideally, the main component of the two-component stream is not adsorbed in an appreciable extent (and may be considered inert) that leads to the achievement of a very good selectivity.
In order to simplify mathematical calculations, some professionals prefer to use the limited selectivity (varying in the range from 0 to 1), then there are used the ratio inverse to that described above. Therefore, when discussing the adsorption processes, it is important to clarify the definition immediately, or at least to abandon the adsorption characteristics such as high or low (large or small), and use the terms good or bad.
In cases when adsorption is carried out at a temperature or pressure changes, a high adsorption capacity may be less preferable than a good selectivity. This is due to the fact that adsorbents with a high adsorption capacity usually poorly to regeneration (hereinafter referred to desorption of the substance adsorbed on the adsorbent, not restoring its properties lost due to coke deposition or by the action of poisons).
The capacity for regeneration is essential when using any cyclic adsorption plants, as in all successive cycles of the adsorbent-enforcement should work with the same efficiency. This means that each component must adsorb sufficiently weak, rather subjected to physical adsorption, but not chemisorptions. A measure of the energy required for regeneration is discussed below particular option – the heat of adsorption. From the standpoint of recovery efficiency, low values for this parameter are preferred.
Regeneration may be carried out by changing the temperature (thermal setup variables) or the pressure (unit with variable pressure) or chemical means – via displacement elution or supercritical extraction. Sometimes, a combination of these methods applies.
Method of displacement is introduction of regenerant, i.e. a substance which adsorbs more strongly than the substance previously adsorbed. Elution is a dissolution of adsorbed material in a solvent which itself is adsorbed on the adsorbent adsorb poorly or not at all. In all cases, the use of chemical methods requires an additional process step to ensure the recovery of the regenerating substance, and it can be an expensive operation. Furthermore, there is the need to use special devices to purge a regenerating bed of substance.
The adsorbent may be regenerated and in contact with the flow in a phase that is different from that in which the adsorption is carried out (for example, steam regeneration after adsorption in the liquid phase). This requires draining or displacement of the previous phase, and therefore additional time, so the use of such methods should be avoided as much as possible.
Share of the adsorbed material that can be extracted during the regeneration (sometimes called the working adsorption capacity), as well as necessary for the regeneration a time and energy is determined by the degree regenerability adsorbent. Often during the first few cycles a short-term drop in the value of the working adsorption capacity provides. During this fall, a few hundred cycles, should be a gradual decline in the value of this parameter is associated with aging, poisoning of the adsorbent or other causes unrelated to the considered recovery which, however, affect the service life adsorbent. It deals only with those cases where the spent adsorbent is regenerated without overloading other devices. This principle is used in most modern techniques of adsorption. Overload in other apparatus or installation is usually required only for regeneration of the adsorbent – activated carbon.
Kinetic parameters of mass transfer are associated with the mass transfer resistance inside the particles. The value of this parameter is large enough, as it is determined by the duration of the operating cycle in adsorption technology using a fixed bed of adsorbent. With the rapid flow of the adsorption processes of change in time of the concentration of the adsorbed substance in the effluent stream from the column will correspond to a constant level as long as the adsorbent is saturated almost completely, and then this level will go up dramatically.
Such dependence on a preferred practice, the curve is called a narrow slip (Figure 2, curve 1). The reverse situation, when the slow flow adsorption processes results in concentration in the effluent stream begins to change shortly after the beginning of the adsorption cycle gives the relationship, which is called a wide breakthrough curve (Figure 2, curve 2).
In the latter case, the situation can be corrected by adding an additional quantity of adsorbent, or increasing the cycle time (which reduces the capacity per unit weight or volume of the adsorbent). Wherein for the second variant also requires a larger amount of adsorbent. For the compensation of the slow adsorption, moreover, can be used with adsorbent particles finer grind (such method is described below in detail), but this is accompanied by a corresponding jump in pressure drop.
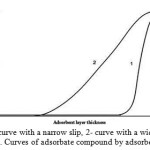 |
Figure 2: Curves of adsorbate compound by adsorbent layer
|
Kinetic parameters sometimes are on the principle of adsorption separation method. For example, in some systems with variable pressure to extract nitrogen from air using carbon molecular sieve adsorbents, in which the rate of adsorption of oxygen is significantly higher than that of nitrogen. However, the conventional disadvantage of this method is the slow diffusion of a given adsorbate (adsorbed substances).
In assessing the suitability of an adsorbent is necessary to consider the possible consequences of chemical and physical influences on it that can lead to a reduction in the projected life of the adsorbent. As an example of the results of such actions can be mentioned attrition of the adsorbent and biological contamination. The substances constituting the adsorbent particles, including such as a binder or a surface modifier containing molecules reactive functional group should be inert to the components of both the feed and regeneration streams. Should not be a reason of unwanted disintegration of adsorbent particles and operating conditions such as flow rate, temperature, pressure or vibration of the equipment.
The problems of the suitability of an adsorbent should be treated very carefully, because it is obvious they do not always. For example, ketones containing streams passing through the adsorption of activated carbon fibers, capable of causing oxidation of the latter. However, new adsorbents have been developed that are resistant to oxidation under these conditions.
Regulation of the rheological properties in the preparation of highly porous adsorbents tend to provide a variety of complex techniques: the external influences on the structured blend, particularly induced vibration the thixotropy effect, and the introduction of surfactants, temperature changes etc. The necessity for such methods combinations determined by the fact that none of the conventional methods are not without drawbacks [4-6].
The basis of porogen removal laid evaporation or burning of blowing agent that occurs when a medium or a high temperature exposure. As a blowing agent water is used, volatile liquid, burnable solid additives. At the using formed burnout agents typically porous honeycomb structure, when using volatile liquids – a capillary-porous.
If it is known the mass concentration of blowing agent in the material and the mass concentration of nucleating material, the porosity of the formed material can be calculated using the formula:

where pp and pm– the density of a blowing agent and nucleating agent, respectively.
The method of leaky packing is applied during the manufacture of fibrous and granular highly porous materials. In application to fibrous materials this method based on felting ability, i.e. for entangling of fibers and keeping of the shape retention due to the friction and hooking between the fibers [3]. In result the obtained porous fibrous structure characteristics depend on the thickness and length of the fibers used, and the preservation of original properties – of elastic fibers. Increasing the total porosity determined by primarily using monofractional composition grains and decreasing the pore size characteristic reduction in their average size.
Materials and Methods
As the study object was selected kulantau vermiculite that represents micaceous magnesium-aluminum silicate glandular of the intermittent chemical composition expanding the structural unit relating to the group trioctahedral hydromicas [3-4].
Vermiculite is mineral from the group of hydrous layered structure, the grain structure of the plate, shiny, can be gold, silver, red ottenkov. At the heating till different temperatures in the 400-1000 °C, natural vermiculite expands 15-25 times and is converted into expanded vermiculite, which is widely used in various sectors of industrial activity.
Characteristic properties of vermiculite:
- specific weight – 70-180 kg/m3 (depending on granules sizes)
- water absorption capacity ~ 400-530%
- pH 6.8-7.0 (neutral-weakly alkaline)
Vermiculites variable chemical composition,%: MgO 14-15, FeO 1-3, Fe2O3 3-17, Al2O3 10-17, SiO2 34-42, H2O 8-15, as well as impurities such as a Ti, Ni , Zn, Cu, Na, K.
The interlayer and inter-packet intervals vermiculite structure can be regarded as plate micropores having a size of 0.3 – 1.2 nm [5]. Vermiculite cation exchange capacity is in the range of 100-150 mEq/100 g, i.e. clay minerals from it one of the most exchange capable [3].
 |
Figure 3: Photo of the kulantau vermiculite sample |
The physicochemical methods of research were used. The structure of the expanded vermiculite was investigated by X-ray method on the DRON-3 [7-8].
Results and Discussion
One of the most common ways is the swelling. This method porization based on isolation in the plastic-viscous mass or introducing into it a gas phase as hydrogen, oxygen, carbon dioxide, water vapor, air, pentane, freon, etc. As a result of the saturation mass of the gas phase increases its volume – occurs swelling (foaming). Formed dispersion – air “fluid” hardening at further processed. When swelling is formed honeycomb porous structure, total porosity that depends on the number entered, and keep the masses of gaseous component. The rheological characteristic of porous masses has a decisive effect on the porous structure. Common for all versions is a swelling plastic-viscous state of masses porization during their porization, i. e. masses porization must be able to permanently deform (flow) without discontinuity. There is only one material swelling which occurs without his move to plastic-viscous (pyroplastic) state – vermiculite [9,10]. This formed plate porosity due separation mica platelets inter-packet with water, turning into vapor state by heating the vermiculite particles to high temperatures.
Therefore, the description of the structure of a porous material based on vermiculite requires the use of dynamic models that include the stage of evaporation in inter-packet space, and reorientation of mica elements under pressure generated steam. In this regard, vermiculite temporal evolution of the porosity of the material can be described with the swelling logistical function of the type:

where
ε0 – the initial porosity until the swelling process;
ε1 – “resource” of porosity due to the reorientation, and the parting of the buckling of plates of mica;
t- is the characteristic time of intense swelling, determines the intensity of the thermal regime.
For the mono factional granular materials, one of three types of packaging implements: octahedral, tetrahedral and rhombohedral [11].
If the number of layers of packaging octahedral grains per unit equal to the thickness of the Mother, the specific porosity of the layer is determined by the formula:

At the using a regular pattern of fibrous materials styling gives an adequate description of the structure of the porous layer. It is found that the fluctuation of the porosity in this case with good accuracy by the Gaussian with a mean porosity:
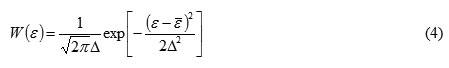
In the case of a porous binder material penomassy obtained honeycomb porosity, the porosity grain emerging from the binder and porosity:
![]()
The research results of the internal structure of adsorption layers means the adsorption isotherms leads to the conclusion that the internal surface of the porous layer is characterized by an extremely complex and developed form can be described by means of fractal geometry [9-11].
At the same time the inner surface layer is characterized by the fractal dimension is higher than “normal” geometric dimension of the surface:
![]()
The specific value of the porosity and internal surface depends on the geometrical characteristics of the primary layer, i.e. granule size and the method of their placement and size of the molecules in the layer are deposited.
The characteristic size of the swelling is in the order of the average grain size ~. At length scale defined by the smallest area of adsorption
![]()
the amount of substance in the adsorbed layer with a fractal surface will vary according to the law:

Thus the main question is reduced to the determination of the characteristics of two layers: minimal surface adsorption, which is connected with the porous layer, and the fractal dimension of which depends on the size and method of stacking the granules. It can be following evaluation.
Let – some characteristic radius of the sphere around the seed. This size is determined so as to accommodate the size of grains, a layer of material adsorbed thereon and the neighboring grain adsorbent in contact with the grain. Then the characteristic volume is usually defined as:
V˜R3 (9)
And to assess the characteristic free surface around the grain fractal adsorbent bed can be written the relation:

Hence we obtain the expression for the specific surface area:
![]()
Estimates for the radius can be drawn from the following considerations.
Considering approximately grains as an ellipsoid stirrup characteristic dimensions, will be obtained for its volume:

It is necessary to consider an amendment to the amount of the adsorbed monolayer of molecules:

where F- the form factor of the grain.
Then is obtained from the equality condition of the hypothetical sphere to the volume V+ΔV or.

Evaluation of the porosity of the adsorbent plate adsorbent, in particular vermiculite considering two characteristic scale can be derived from the following relationship [2,3]:

Further it can get an estimate of the fractal dimension of exfoliated vermiculite. In fact the condition of completion of the swelling t≈t:
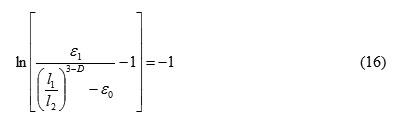
In result:

In general, a porous layer is a cluster that characterized by two parameters: the average radius of advanced adsorption ÝN and width of the core Ψ For a large numbers of components of the particle layer of fractal theory to the relation:
![]()
where γ- the measure, depending on the characteristic length.
The corresponding expression for the width of the core also gives the fractal theory, the average distance between the branches of the cluster:

where χ- the index of the inner layer of the anisotropy of the adsorbent.
Thickness of the adsorbent layer may be used for the global characteristic quantity of a length.
Conclusion
The fractal dimension of the cluster is determined by the physicochemical characteristics and the conditions of formation of a porous layer. A critical component and the degree of anisotropy may depend on the physicochemical characteristics of the reactants and the geometric characteristics of the layer. Unusual flow regimes of complex multicomponent fluids in porous media appear in a nonuniform distribution of the adsorbed substance in the adsorbed layer.
The presented information in the model can be used in the organization of systematic experimental studies of porization and adsorption of highly adsorptive layers, as well as for the optimization of porization materials.
References
- Gelperin N.I. Basic processes and apparatuses of chemical technology. Moscow, Chemistry, 1981. 812.
- Greg S., Singh K. Adsorption, surface area, porosity. Moscow, Mir, 1970. 407p.
- Syrmanova K.K., Zh.B.Kaldybekova. Polyfunctional sorbents. Monograph, Sh., 2012.168.
- Syrmanova K., Kaldybekova Zh., Sakibaeva S., Brener A. Expanded Vermiculite Based Adsorbent. Journal of Materials Science and Engineering. USA, 2012, 2(4),. 313-316.
- Fedorov N.F., Sevryugov L.V. Adsorbents and adsorption processes. Kishinev, 1986,. 117-118.
- Gorelik S.S., Rastorgouev L.N., Skakov Y.A. X-ray and electron-optical analysis. Publishing house. Moscow, Metallurgy, 1990, 260.
- Ed. Brown, X-ray techniques to study the structure of clay minerals Moscow, Mir, 1995, 599.
- Syrmanova K.K., Kaldybekova J.B. Perspectives for the use of vermiculite in South Kazakhstan [Collected scientific works of the IV International scientific and practical conference “Effective constructions. Theory and practice”]. Penza, 2005,. 323-327.
- Kaldybekova J.B., Syrmanova K.K., Brener A.M. Mathematical modeling of the formation of structure in the swelling high porosity materials [Proceedings of the XV International Conference on Computing Mechanics and Advanced Applied Systems (VMSPPS’ 2007)]. Russia, Alushta. 2007, 252-253.
- Kaldybekova J.B., Brener A.M., Syrmanova K.K. Simulation of two-phase fluid flow in a layer of porous adsorbent. [Bulletin of the National Academy of Sciences of the Republic of Kazakhstan], 2008,.1,.9-12.
- Syrmanova K., Negim E., Kaldybekova J., Tuleuov A.M. Epoxylitane Сompositions Modification with Using Thermoplastic Polyurethane Orien J Chem 2016 32(1)..1-7

This work is licensed under a Creative Commons Attribution 4.0 International License.









