One-pot and Three-Component Synthesis of isoxazol-5(4H)-one Derivatives in the presence of Citric Acid
Ashkan bashash Rikani and Davood Setamdideh*
Department of Chemistry, Mahabad Branch, Islamic Azad University, Mahabad, Iran.
Corresponding Author E-mail: davood.setamdideh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320317
Article Received on : February 02, 2016
Article Accepted on : March 06, 2016
In this context, the one-pot three-component synthesis of 3-methyl-4-arylmethylene-isoxazol-5(4H)-ones has been performed in the presence of citric acid as catalyst in water. The products have been obtained in high yields (70-90%) and convenient reaction times (5-24 hours).
KEYWORDS:Citric Acid; isoxazol-5(4H)-one; one-pot reaction; water; green chemistry
Download this article as:| Copy the following to cite this article: Rikani A. B, Setamdideh D. One-pot and Three-Component Synthesis of isoxazol-5(4H)-one Derivatives in the presence of Citric Acid. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Rikani A. B, Setamdideh D. One-pot and Three-Component Synthesis of isoxazol-5(4H)-one Derivatives in the presence of Citric Acid. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=17976 |
Introduction
Isoxazolones and its derivatives have a variety of biological activities. 1 The synthesis of aryl-3-methylisoxazol-5(4H)-one derivatives involves a coupling of aromatic aldehydes with ethyl acetoacetate and hydroxylamine. This procedure has been carried out by different reagents and catalysts .2 Also, multi-component reactions (MCRs) have been used for the synthesis of a variety of natural products and biologically active compounds, because they have many advantages such as excellent functional group compatibility, minimization of waste, versatility, atom economy, environmentally friendly, and easy work-up [3]. In this context we have done a convenient and environmentally benign procedure by citric acid as the catalyst for the synthesis of 4-arylmethylidene-3-substituted-isoxazol-5(4H)-ones. Thus, the synthesis of 4-arylmethylidene-3-methyl-isoxazol-5(4H)-ones was attempted by using equimolecular quantities of ethyl acetoacetate, hydroxylamine hydrochloride, and a variety of aromatic aldehydes in the presence of citric acid as catalyst in water as shown in scheme 1.
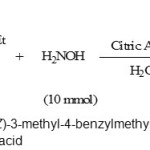 |
Scheme 1: The synthesis of (Z)-3-methyl-4-benzylmethylene-isoxazol-5(4H)-ones in the presence of citric acid Click here to View Scheme |
Results and Discussion
We conceived the preparation of diverse arylmethylidene-isoxazole-5(4H)-ones from the reaction between an aromatic aldehyde, hydroxylamine hydrochloride, and ethyl acetoacetate (EAA) catalyzed by citric acid. For determine the optimal reaction conditions, we screened different amounts of citric acid (0-2 mmol) (Table 1) using benzaldehyde as a model compound. Consequently, the amount of 1 mmol for citric acid was selected as the optimized amount of the catalyst for this procedure.
| Table 1: Optimization reaction condition for the synthesis of (Z)-4-benzylidene-3-methylisoxazol-5(4H)-one (5a) from benzaldehyde (10 mmol), ethyl acetoacetate (10 mmol) and NH2OH.HCl (10 mmol) in H2O (10 ml) in the presence of Citric Acid as shown in scheme 1. | ||||
|
Entry |
Citric Acid (g) |
time (min) |
conversion (%)a |
yield (%)b |
|
1 |
0 |
10 |
100< |
30 |
|
2 |
0.25 |
10 |
100< |
50 |
|
3 |
0.5 |
10 |
100< |
70 |
|
4 |
1 |
8 |
100 |
90 |
|
5 |
2 |
7 |
100 |
90 |
| a Conversion refers to TLC monitoring. b Yield refers to isolated pure product. | ||||
The efficiency of this protocol was examined by the reaction of a variety of aldehydes. all reactions were completed in appropriate times within 5-24 hours in good yields (70-90%) (Table 2).
| Table 2: Synthesis of (Z)-4-arylmethylidene-3-methyl-isoxazol-5(4H)-ones with Citric Acid/H2O. | ||||
|
Entry |
Product |
time (h) |
yield (%) a |
mp (°C) b |
|
1 |
Ph |
8 |
90 |
140-142 |
|
2 |
4-MeO-Ph |
5 |
90 |
177-179 |
|
3 |
2-MeO-Ph |
5 |
85 |
159-160 |
|
4 |
Ph-CH=CH |
6 |
96 |
180-182 |
|
5 |
3-Br-Ph |
24 |
70 |
141-143 |
|
6 |
4-Me2N-Ph |
5 |
90 |
206-209 |
|
7 |
4-Me-Ph |
6 |
80 |
129-131 |
| a Yields refer to isolated pure products after recrystallization inappropriate solvent. b The melting points have been compared with the literatures. | ||||
The products were characterized by 1H-NMR spectroscopy, FT-IR spectrum and melting points of the products (Table 2, column 6) were measured and compared with the literature for the known compounds [2]. Characterization and comparison of the formed products with suitable references [2k and 2l] supports the selectivity for the formation of the (Z)-isomer. Therefore, the products are assumed to have the double bond with the (Z) geometry.
Two proposed mechanism for the formation of the products and the influences of citric acid are shown in Schemes 2.
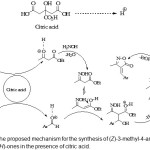 |
Scheme 2: The proposed mechanism for the synthesis of (Z)-3-methyl-4-arylmethylene-isoxazol-5(4H)-ones in the presence of citric acid. Click here to View Scheme |
Experimental
General
All substrates and reagents were purchased from commercially sources (Merck and Sigma-Aldrich). FT-IR, 1H-NMR, and 13C-NMR spectra were recorded on PerkinElmer FT-IR RXI and 300 MHz Bruker spectrometers, respectively. The products were characterized by their FT-IR, 1H-NMR, and 13C-NMR spectra and comparison with authentic samples. Organic layers were dried over anhydrous sodium sulfate. All yields referred to isolated pure products. The purity of products was determinate by 1H NMR. Also, reactions were monitored over silica gel 60 F254 aluminum sheet.
A typical procedure for the synthesis of (Z)-4-arylmethylene-3-methyl-isoxazol-5(4H)-ones
In a round-bottomed flask (25 mL) equipped with a magnetic stirrer, a mixture of ethyl acetoacetate (1.30 g, 10 mmol), hydroxylamine hydrochloride (0.7 g, 10 mmol), aromatic aldehyde (10 mmol), and citric acid (1.9 g, 10 mmol) in 25 mL of distilled water was prepared and stirred at room temperature for mentioned time in Table 2. After completion of reaction (monitored by TLC), the precipitate was filtered off and washed with cold distilled water. Then products were recrystallized from ethanol or acetone as mentioned in Table 2. Pure (Z)-4-arylmethylene-3-methyl-isoxazol-5(4H)-ones were obtained as solids after recrystallization from ethanol or acetone and were characterized by 1H-NMR, 13C-NMR, and FT-IR spectroscopy.
Spectral data for prepared compounds
(Z)-4-benzylidene-3-methylisoxazol-5(4H)-one (1)
Yellow crystal: mp 140-142 °C (Lit [2k] mp 142-144 °C);1H-NMR (300 MHz, CDCl3): δ 2.31 (s, 3H, CH3), 7.44 (s, 1H, ArCH=), 7.49-7.59 (m, 3H, Ar), 8.35 (dd, J = 1.3, 7.4 Hz, 2H, Ar); 13C-NMR (300 MHz, CDCl3): δ 11.63 (CH3), 119.65 (C=, inside of isoxazolone ring), 129.03 (Ar), 130.47 (Ar), 132.29 (Ar), 134.01 (Ar), 149.98 (ArCH=), 161.16 (C=N), 167.88 (C=O); IR (KBr) ν: 1732 (C=O), 1620, 1100, 1216, 879, 763 cm−1.
(Z)-4-(4-methoxybenzylidene)-3-methylisoxazol-5(4H)-one (2)
Yellow crystal: mp 177-179 °C (Lit [2k] mp 177-178 °C);1H-NMR (300 MHz, CDCl3): δ 2.28 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 7.34 (s, 1H, ArCH=), 7.00 (d, J = 8.7 Hz, 2H, Ar), 8.44 (d, J = 8.7 Hz, 2H, Ar); 13C-NMR (300 MHz, CDCl3): δ 11.63 (CH3), 55.70 (OCH3), 114.64 (C=, inside of isoxazolone ring), 116.31 (Ar), 125.82 (Ar), 136.96 (Ar), 149.35 (ArCH=), 161.29 (Ar-O), 164.60 (C=N), 168,77 (C=O); IR (KBr) ν: 1730 (C=O), 1590, 1267, 1018, 875, 775 cm−1.
(Z)-4-(2-methoxybenzylidene)-3-methylisoxazol-5(4H)-one (3)
Yellow crystal: mp 159-160 °C; 1H-NMR (300 MHz, CDCl3): δ 2.31 (s, 3H, CH3), 3.95 (s, 3H, OCH3), 6.96 (d, J = 8.4 Hz, 1H, Ar), 7.09 (t, J = 7.8 Hz, 1H, Ar), 7.56 (t, J = 7.05 Hz, 1H, Ar), 8.06 (s, 1H, ArCH=), 8.92 (d, J = 8.1 Hz, 1H, Ar); 13C-NMR (300 MHz, CDCl3): δ 11.67 (CH3), 55.47 (OCH3), 110.70 (C=, inside of isoxazolone ring), 118.32 (Ar), 120.84 (Ar), 121.20 (Ar), 133.37 (Ar), 136.27 (Ar), 143.98 (ArCH=), 159.82 (C=N), 161.52 (C=O); IR (KBr) ν: 1732 (C=O), 1590, 1256, 1103, 887, 765 cm−1. IR (KBr) ν: 1732 (C=O), 1590, 1256, 1103, 887, 765 cm−1.
(Z)-3-methyl-4-(3-phenylallylidene)isoxazol-5(4H)-one (4)
Yellow crystal: mp 180-182 °C (Lit [2k] mp 179-181 °C); 1H-NMR (300 MHz, CDCl3): δ 2.25 (s, 3H, CH3), 7.28-7.36 (m, 2H, CH=CH), 7.36-7.47 (m, Ar (2H) & ArCH= (1H)), 7.64-7,66 (m, 2H, Ar), 8.26-8.35 (m, 1H, Ar); 13C-NMR (300 MHz, CDCl3): δ 11.19 (CH3), 117.86 (C=, inside of isoxazolone ring), 121.34 (Ar), 122.38 (Ar), 129.31 (Ar), 131.53 (C=C), 134.96 (C=C), 147.53 (Ar), 151.45 (ArCH=), 159.89 (C=N), 168.99 (C=O); IR (KBr) ν: 1733 (C=O), 1542, 1103, 993, 848, 753 cm−1.
(Z)-4-(3-bromobenzylidene)-3-methylisoxazol-5(4H)-one (5)
Yellow crystal: mp 141-143 °C; 1H-NMR (300 MHz, CDCl3): δ 2.31 (s, 3H, CH3), 7.35 (s, 1H, ArCH=), 7.41 (t, J = 8.1 Hz, 1H, Ar), 7.71 (d, J = 7.80 Hz, 1H, Ar), 8.34 (d, J = 7.8 Hz, 1H, Ar), 8.46 (s, 1H, Ar); 13C-NMR (300 MHz, CDCl3): δ 11.60 (CH3), 121.12 (Ar-Br), 122.89 (C=, inside of isoxazolone ring), 130.48 (Ar), 131.90 (Ar), 133.87 (Ar), 136.03 (Ar), 136.50 (Ar), 147.71 (ArCH=), 160.86 (C=N), 167.45 (C=O); IR (KBr) ν: 1729 (C=O), 1544, 1217, 1123, 871, 775 cm−1.
(Z)-4-(4-methylbenzylidene)-3-methylisoxazol-5(4H)-one (6)
Lemon crystal: mp 129-131 °C (Lit [2j] mp 126-127 °C); 1H-NMR (300 MHz, CDCl3): δ 2.29 (s, 3H, CH3), 2.45 (s, 3H, CH3), 7.32 (d, J = 7.8 Hz, 2H, Ar), 7.40 (s, 1H, ArCH=), 8.29 (d, J = 7.8 Hz, 2H, Ar); 13C-NMR (300 MHz, CDCl3): δ 11.65 (CH3), 22.07 (CH3), 118.40 (C=, inside of isoxazolone ring), 129.88 (Ar), 134.14 (Ar), 145.73 (Ar), 149.96 (ArCH=), 161.22 (C=N), 168.21(C=O); IR (KBr) ν: 1731 (C=O), 1594, 1114, 873, 777 cm−1.
(Z)-4-(4-(dimethylamino)benzylidene)-3-methylisoxazol-5(4H)-one (7)
Red crystal: mp 206-209 °C (Lit [2k] mp 206-207 °C); 1H-NMR (300 MHz, CDC13): δ 2.23 (s, 3H, CH3), 3.15 (s, 6H, N(CH3)2), 6.71 (d, J = 9 Hz, 2H, Ar), 7.27 (s, 1H, ArCH=), 8.39 (d, J = 9 Hz, 2H, Ar); 13C-NMR (300 MHz, CDCl3): δ 11.71 (CH3), 40.10 ((CH3)2N), 111.07 (Ar), 111.50 (C=, inside of isoxazolone ring), 121.51 (Ar), 137.62 (Ar), 149.26 (Ar), 154.22 (ArCH=), 161.59 (C=N), 170.12 (C=O); IR (KBr) ν: 1714 (C=O), 1557, 1380, 1196, 1095, 867, 765 cm−1.
Conclusion
In conclusion, we have shown that citric acid in water is a convenient catalyst for the preparation of a variety of alkylidene isoxazol-5(4H)-ones, using aromatic aldehydes, ethyl acetoacetate, and hydroxylamine hydrochloride precursors in one-pot, three-component condensation reaction at room temperature in excellent yields. High efficiency, convenient reaction times, easy work-up, mild reaction conditions, and using of water as a green solvent make to this new protocol attractive for the synthesis of these heterocycles.
Acknowledgments
The authors gratefully appreciated the financial support of this work by the research council of Islamic Azad University branch of Mahabad.
References
- a) Knecht, W.; Löffler, M. Biochem. Pharmacol. 1998, 56, 1259-1264. b) Suryawanshi, S. N.; Tiwari, A.; Chandra, N.; Ramesh.; Gupta, S. Bioorg. Med. Chem. Lett. 2012, 22, 6559-6562. c) Kafle, B.; Cho, H. Bull. Korean Chem. Soc. 2012, 33, 275-277. d) Changtam, C.; Hongmanee, P.; Suksamrarn, A. Eur. J. Med. Chem. 2010, 45, 4446-4457. e) Santos, M. M. M.; Faria, N.; Iley, J.; Coles, S. J.; Hursthouse, M. B.; Martins, M. L.; Moreira, R. Bioorg. Med. Chem. Lett. 2010, 20, 193-195. f) Conti, P.; Tamborini, L.; Pinto, A.; Sola, L.; Ettari, R.; Mercurio, C.; De Micheli, C. Eur. J. Med. Chem. 2010, 45, 4331-4338. g) Kano, H.; Adachi, I.; Kido, R.; Hirose, K. J. Med. Chem. 1967, 10, 411-418. h) Giovannoni, M. P.; Vergelli, C.; Ghelardini, C.; Galeotti, N.; Bartolini, A.; Piaz, V. D. J. Med. Chem. 2003, 46, 1055-1059. i) Srinivas, A.; Nagaraj, A.; Reddy, C. S. Eur. J. Med. Chem. 2010, 45, 2353-2358. j) Padmaja, A.; Rajasekhar, C.; Muralikrishna, A.; Padmavathi, V. Eur. J. Med. Chem. 2011, 46, 5034-5038. k) Padmaja, A.; Payani, T.; Reddy, G. D.; Padmavathi, V. Eur. J. Med. Chem. 2009, 44, 4557-4566. l) Prashanthi, Y.; Kiranmai, K.; Subhashini, N. J. P.; Shivaraj. Spectrochim. Acta A. 2008, 70, 30-35. m) Talley, J. J.; Brown, D. L.; Carter, J. S.; Graneto, M. J.; Koboldt, C. M.; Masferrer, J. L.; Perkins, W. E.; Rogers, R. S.; Shaffer, A. F.; Zhang, Y. Y.; Zweifel, B. S.; Seibert, K. J. Med. Chem. 2000, 43, 775-777. n) Karabasanagouda, T.; Adhikari, A. V.; Girisha, M. Indian J. Chem. B 2009, 48, 430-437. o) Kamal, A.; Bharathi, E. V.; Reddy, J. S.; Janaki, M.; Ramaiah, D.; Reddy, M. K.; Viswanath, A.; Reddy, T. L.; Shaik, T. B.; Pushpavalli, S. N.; Bhadra, M. P. Eur. J. Med. Chem. 2011, 46, 691-703. p) Lee, Y. S.; Park, S. M.; Kim, B. H. Bioorg. Med. Chem. Lett. 2009, 19, 1126-1128. q) Mao, J.; Yuan, H.; Wang, Y.; Wan, B.; Pak, D.; He, R.; Franzblau, S. G. Bioorg. Med. Chem. Lett. 2010, 20, 1263-1268. r) Tavares, A.; Arruda, B. C.; Boes, E. L.; Stefani, V.; Stassen, H. K.; Campo, L. F.; Bechtoldb, I. H.; Merlo, A. A. J. Braz. Chem. Soc. 2012, 23, 880-888. s) Zhang, X. H.; Wang, L. Y.; Zhan, Y. H.; Fu, Y. L.; Zhaia, G. H.; Wenc, Z. Y. J. Mol. Struct. 2011, 994, 371-378. t) Zhang, X. H.; Zhan, Y. H.; Chen, D.; Wang, F.; Wang, L. Y. Dyes Pigments 2012, 93, 1408-1415. u) Pu, S.; Li, H.; Liu, G.; Liu, W.; Cui, S.; Fan, C. Tetrahedron 2011, 67, 1438-1447. v) Liu, G.; Liu, M.; Pu, S.; Fan, C.; Cui, S. Tetrahedron 2012, 68, 2267-2275.
- a) Zhang, Y. Q.; Ma, J. J.; Wang, C.; Li, J. C.; Zhang, D. N.; Zang, X. H.; Li, J. Chin. J. Org Chem. 2008, 28, 141-144. b) Liu, Q.; Wu, R. T. J. Chem. Res. 2011, 10, 598-599. c) Liu, Q.; Zhang, Y. N. Bull. Korean Chem. Soc. 2011, 32, 3559-3560. d) Kiyani, H.; Ghorbani, F. Elixir Org. Chem. 2013, 58A, 14948-14950. e) Kiyani, H.; Ghorbani, F. Heterocycl Lett. 2013, 3, 359-369. f) Kiyani, H.; Ghorbani, F. Heterocycl Lett. 2013, 3, 145-153. g) Liu, Q.; Hou, X. Phosphorus, Sulfur Silicon Relat. Elem. 2012, 187, 448-453. h) Kiyani, H. Org. Chem. Indian J. 2013, 9, 97-101. i) Kiyani, H.; Ghorbani, F. Open J. Org. Chem. 2013, 1, 5-9. j) Zhang, Y. Q.; Wang, C.; Zhang, M. Y.; Cui, P. L.; Li, Y. M.; Zhou, X.; Li, J. C. Chin. J. Org. Chem. 2008, 28, 914-917. k) Cheng, Q. F.; Liu, X. Y.; Wang, Q. F.; Liu, L. S.; Liu, W. J.; Lin, Q.; Yang, X. J. Chin. J. Org. Chem. 2009, 29, 1267-1271. l) Saikh, F.; Das, J.; Ghosh, S. Tetrahedron Lett. 2013, 54, 4679-4682. m) Fozooni, S.; Gholam Hosseinzadeh, N.; Hamidian, H.; Akhgar, M. R. J. Braz. Chem. Soc. 2013, 24, 1649-1655. n) Chavan, A. P.; Pinjari, A. B.; Mhaske, P. C. 2014, J. Heterocyclic Chem. doi: 10.1002/jhet.2293.
CrossRef - a) Singh, M. S.; Chowdhry, S. RSC Adv. 2012, 2, 4547-4592. b) Murthy, S. N.; Madhav, B.; Kumar, A. V.; Rao, K. R.; Nageswar, Y. V. D. Helv. Chem. Acta 2009, 92, 2118-2124. c) Jiang, B.; Wang, X.; Shi, F.; Tu, S. J.; Li, G. Org. Biomol. Chem. 2011, 9, 4025-4028. d) Wan, J. P.; Liu, Y. RSC Adv. 2012, 2, 9763-9777. e) Dӧmling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083-3135. f) Ramόn, D. J.; Yus, M. Angew. Chem. Int. Ed. 2005, 44, 1602-1634. g) de Graaff, C.; Ruijter, E.; Orru, R. V. A. Chem. Soc. Rev. 2012, 41, 3969-4009. h) Ugi, I. Pure Appl. Chem. 2001, 73, 187-191. i) Kumaravel, K.; Vasuki, G. Curr. Org. Chem. 2009, 13, 1820-1841. j) Chanda, A.; Fokin, V. V. Chem. Rev. 2009, 109, 725-748. k) Gu, Y. Green Chem. 2012, 14, 2091-2128. l) Siamala, M. Org. Prep. Proced. Int. 2009, 41, 1-68.

This work is licensed under a Creative Commons Attribution 4.0 International License.









